Single-cell RNA sequencing to decipher the immunogenicity of ChAdOx1 nCoV-19/AZD1222 and mRNA-1273 vaccines in patients with autoimmune rheumatic diseases
- PMID: 35979368
- PMCID: PMC9376226
- DOI: 10.3389/fimmu.2022.920865
Single-cell RNA sequencing to decipher the immunogenicity of ChAdOx1 nCoV-19/AZD1222 and mRNA-1273 vaccines in patients with autoimmune rheumatic diseases
Abstract
Objectives: To investigate the differences between the vector vaccine ChAdOx1 nCoV-19/AZD1222 (Oxford-AstraZeneca) and mRNA-based vaccine mRNA-1273 (Moderna) in patients with autoimmune rheumatic diseases (AIRD), and to explore the cell-cell interactions between high and low anti-SARS-CoV-2 IgG levels in patients with rheumatic arthritis (RA) using single-cell RNA sequencing (scRNA-seq).
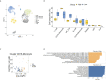
Methods: From September 16 to December 10, 2021, we consecutively enrolled 445 participants (389 patients with AIRD and 56 healthy controls), of whom 236 were immunized with AZD1222 and 209 with mRNA-1273. The serum IgG antibodies to the SARS-CoV-2 receptor-binding domain was quantified by electrochemiluminescence immunoassay at 4-6 weeks after vaccination. Moreover, peripheral blood mononuclear cells (PBMCs) were isolated from RA patients at 4-6 weeks after vaccination for scRNA-seq and further analyzed by CellChat. ScRNA-seq of PBMCs samples from GSE201534 in the Gene Expression Omnibus (GEO) database were also extracted for analysis.
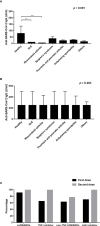
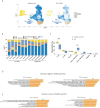
Results: The anti-SARS-CoV-2 IgG seropositivity rate was 85.34% for AIRD patients and 98.20% for healthy controls. The anti-SARS-CoV-2 IgG level was higher in patients receiving mRNA-1273 than those receiving AZD1222 (β: 35.25, 95% CI: 14.81-55.68, p=0.001). Prednisolone-equivalent dose >5 mg/day and methotrexate use in AIRD patients, and non-anti-tumor necrosis factor-α biologics and Janus kinase inhibitor use in RA patients were associated with inferior immunogenicity. ScRNA-seq revealed CD16-monocytes were predominant in RA patients with high anti-SARS-CoV2-IgG antibodies, and enriched pathways related to antigen presentation via MHC class II were found. HLA-DRA and CD4 interaction was enhanced in high anti-SARS-CoV2-IgG group.
Conclusions: mRNA-1273 and AZD1222 vaccines exhibited differential immunogenicity in AIRD patients. Enriched pathways related to antigen presentation via MHC class II in CD16-monocytes might be associated with higher anti-SARS-CoV2-IgG level in RA patients and further study is warranted.
Keywords: COVID-19 vaccine; anti-rheumatic medications; autoimmune; rheumatic disease; single-cell RNA sequencing.
Copyright © 2022 Chen, Cheng, Huang, Chen, Chen, Chen, Lin, Tang, Hung, Hsieh, Chen, Chen and Hsiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Mehrmal S, Uppal P, Nedley N, Giesey RL, Delost GR. The global, regional, and national burden of psoriasis in 195 countries and territories, 1990 to 2017: A systematic analysis from the global burden of disease study 2017. J Am Acad Dermatol (2021) 84:46–52. doi: 10.1016/j.jaad.2020.04.139 - DOI - PubMed
-
- Rutherford MA, Scott J, Karabayas M, Antonelou M, Gopaluni S, Gray D, et al. UK And Ireland vasculitis rare disease group (UKIVAS). risk factors for severe outcomes in patients with systemic vasculitis and COVID-19: A binational, registry-based cohort study. Arthritis Rheumatol (2021) 73:1713–9. doi: 10.1002/art.41728 - DOI - PMC - PubMed
-
- Gisondi P, Piaserico S, Naldi L, Dapavo P, Conti A, Malagoli P, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: A northern Italy experience. J Allergy Clin Immunol (2021) 147:558–560.e1. doi: 10.1016/j.jaci.2020.10.032 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

