Platelets in the NETworks interweaving inflammation and thrombosis
- PMID: 35979369
- PMCID: PMC9376363
- DOI: 10.3389/fimmu.2022.953129
Platelets in the NETworks interweaving inflammation and thrombosis
Abstract
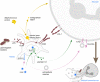
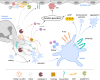
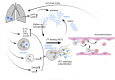
Platelets are well characterized for their indispensable role in primary hemostasis to control hemorrhage. Research over the past years has provided a substantial body of evidence demonstrating that platelets also participate in host innate immunity. The surface expression of pattern recognition receptors, such as TLR2 and TLR4, provides platelets with the ability to sense bacterial products in their environment. Platelet α-granules contain microbicidal proteins, chemokines and growth factors, which upon release may directly engage pathogens and/or contribute to inflammatory signaling. Additionally, platelet interactions with neutrophils enhance neutrophil activation and are often crucial to induce a sufficient immune response. In particular, platelets can activate neutrophils to form neutrophil extracellular traps (NETs). This specific neutrophil effector function is characterized by neutrophils expelling chromatin fibres decorated with histones and antimicrobial proteins into the extracellular space where they serve to trap and kill pathogens. Until now, the mechanisms and signaling pathways between platelets and neutrophils inducing NET formation are still not fully characterized. NETs were also detected in thrombotic lesions in several disease backgrounds, pointing towards a role as an interface between neutrophils, platelets and thrombosis, also known as immunothrombosis. The negatively charged DNA within NETs provides a procoagulant surface, and in particular NET-derived proteins may directly activate platelets. In light of the current COVID-19 pandemic, the topic of immunothrombosis has become more relevant than ever, as a majority of COVID-19 patients display thrombi in the lung capillaries and other vascular beds. Furthermore, NETs can be found in the lung and other tissues and are associated with an increased mortality. Here, virus infiltration may lead to a cytokine storm that potently activates neutrophils and leads to massive neutrophil infiltration into the lung and NET formation. The resulting NETs presumably activate platelets and coagulation factors, further contributing to the subsequent emergence of microthrombi in pulmonary capillaries. In this review, we will discuss the interplay between platelets and NETs and the potential of this alliance to influence the course of inflammatory diseases. A better understanding of the underlying molecular mechanisms and the identification of treatment targets is of utmost importance to increase patients' survival and improve the clinical outcome.
Keywords: COVID-19; NETs; immunothrombosis; neutrophil extracellular traps; neutrophils; platelets.
Copyright © 2022 Wienkamp, Erpenbeck and Rossaint.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome.Blood. 2020 Sep 3;136(10):1169-1179. doi: 10.1182/blood.2020007008. Blood. 2020. PMID: 32597954 Free PMC article.
-
Neutrophil extracellular traps induce aggregation of washed human platelets independently of extracellular DNA and histones.Cell Commun Signal. 2018 May 29;16(1):24. doi: 10.1186/s12964-018-0235-0. Cell Commun Signal. 2018. PMID: 29843771 Free PMC article.
-
Hepatic Surgical Stress Promotes Systemic Immunothrombosis That Results in Distant Organ Injury.Front Immunol. 2020 May 22;11:987. doi: 10.3389/fimmu.2020.00987. eCollection 2020. Front Immunol. 2020. PMID: 32528475 Free PMC article.
-
Pathological roles of NETs-platelet synergy in thrombotic diseases: From molecular mechanisms to therapeutic targeting.Int Immunopharmacol. 2025 Jun 26;159:114934. doi: 10.1016/j.intimp.2025.114934. Epub 2025 May 25. Int Immunopharmacol. 2025. PMID: 40418882 Review.
-
Platelets, NETs and cancer.Thromb Res. 2018 Apr;164 Suppl 1:S148-S152. doi: 10.1016/j.thromres.2018.01.049. Thromb Res. 2018. PMID: 29703474 Review.
Cited by
-
Platelets and Thrombotic Antiphospholipid Syndrome.J Clin Med. 2024 Jan 27;13(3):741. doi: 10.3390/jcm13030741. J Clin Med. 2024. PMID: 38337435 Free PMC article. Review.
-
Pathways of Coagulopathy and Inflammatory Response in SARS-CoV-2 Infection among Type 2 Diabetic Patients.Int J Mol Sci. 2023 Feb 21;24(5):4319. doi: 10.3390/ijms24054319. Int J Mol Sci. 2023. PMID: 36901751 Free PMC article. Review.
-
Neutrophils and Neutrophil-Based Drug Delivery Systems in Anti-Cancer Therapy.Cancers (Basel). 2025 Apr 5;17(7):1232. doi: 10.3390/cancers17071232. Cancers (Basel). 2025. PMID: 40227814 Free PMC article. Review.
-
Delayed Immune Response Upon Injury in Diabetic Wounds Impedes Healing.Immun Inflamm Dis. 2025 Feb;13(2):e70142. doi: 10.1002/iid3.70142. Immun Inflamm Dis. 2025. PMID: 39891428 Free PMC article.
-
Role of S100A8/A9 in Platelet-Neutrophil Complex Formation during Acute Inflammation.Cells. 2022 Dec 6;11(23):3944. doi: 10.3390/cells11233944. Cells. 2022. PMID: 36497202 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

