Chronic Cerebral Hypoperfusion Aggravates Parkinson's Disease Dementia-Like Symptoms and Pathology in 6-OHDA-Lesioned Rat through Interfering with Sphingolipid Metabolism
- PMID: 35979400
- PMCID: PMC9377946
- DOI: 10.1155/2022/5392966
Chronic Cerebral Hypoperfusion Aggravates Parkinson's Disease Dementia-Like Symptoms and Pathology in 6-OHDA-Lesioned Rat through Interfering with Sphingolipid Metabolism
Abstract
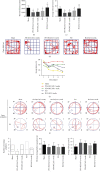
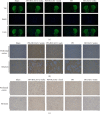
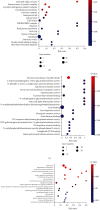
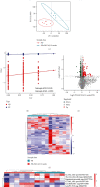
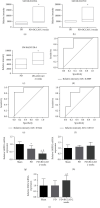
Chronic cerebral hypoperfusion (CCH) is a cardinal risk factor for Parkinson's disease dementia (PDD), but this potential causality lacks mechanistic evidence. We selected bilateral common carotid artery occlusion (BCCAO) to simulate chronic cerebral hypoperfusion in the rat model of PD induced by typical neurotoxin 6-hydroxy dopamine (6-OHDA). Four weeks after unilateral injection of 6-OHDA into the medial forebrain bundle, rats underwent BCCAO. Male Sprague-Dawley rats were divided into five groups of ten, including sham, PD+BCCAO 2 weeks, PD+BCCAO 1 week, PD, and BCCAO 2 weeks. Then, open field test (OFT) and Morris water maze test (MWM) were used to assess the PDD-like symptoms in rats. Also, the pathological manifestations and mechanisms of BCCAO impairing cognitive functions have been explored via hematoxylin-eosin staining, Nissl staining, immunohistochemistry, immunofluorescence, RNA sequencing analysis, lipidomics, and quantitative real-time polymerase chain reaction. In this study, we found that CCH could aggravate PDD-like cognitive symptoms (i.e., learning memory and spatial cognition) and PDD-like pathology (higher expression of α-Syn and Aβ in prefrontal cortex and striatum). Moreover, a potential relationship between differentially expressed mRNAs and lipid metabolism was revealed by RNA sequencing analysis. Lipidomics showed that CCH could affect the intensity of 5 lipids, including sphingomyelin (SM 9:0;2O/26:2; SM 8:1;2O/25:0; and SM 8:0;2O/28:4), cardiolipin, lysophosphatidylcholine, cholesteryl ester, and triacylglycerol. Interestingly, the KEGG pathway analysis of both RNA sequencing analysis and lipidomics suggested that CCH leaded to learning impairment by affecting sphingolipid metabolism. Finally, we found that CCH disrupts the sphingolipid metabolism by affecting the mRNA expression of SMPD1 and SMS2, leading to the accumulation of sphingomyelin in the prefrontal cortex. In summary, CCH, an independent exacerbating reason for impairment in learning and memory within the pathopoiesis of PD, aggravates Parkinson's disease dementia-like symptoms and pathology in 6-OHDA-lesioned rat through interfering with sphingolipid metabolism.
Copyright © 2022 Yaohua Fan et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Huang X., Wang C., Chen L., Zhang T., Leung K. L., Wong G. Human amyloid beta and α-synuclein co-expression in neurons impair behavior and recapitulate features for Lewy body dementia in Caenorhabditis elegans. Biochimica et Biophysica Acta - Molecular Basis of Disease . 2021;1867(10, article 166203) doi: 10.1016/j.bbadis.2021.166203. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

