Integrase deficient lentiviral vector: prospects for safe clinical applications
- PMID: 35979475
- PMCID: PMC9377332
- DOI: 10.7717/peerj.13704
Integrase deficient lentiviral vector: prospects for safe clinical applications
Abstract
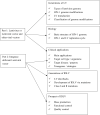
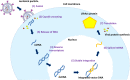
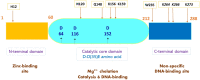
HIV-1 derived lentiviral vector is an efficient transporter for delivering desired genetic materials into the targeted cells among many viral vectors. Genetic material transduced by lentiviral vector is integrated into the cell genome to introduce new functions, repair defective cell metabolism, and stimulate certain cell functions. Various measures have been administered in different generations of lentiviral vector systems to reduce the vector's replicating capabilities. Despite numerous demonstrations of an excellent safety profile of integrative lentiviral vectors, the precautionary approach has prompted the development of integrase-deficient versions of these vectors. The generation of integrase-deficient lentiviral vectors by abrogating integrase activity in lentiviral vector systems reduces the rate of transgenes integration into host genomes. With this feature, the integrase-deficient lentiviral vector is advantageous for therapeutic implementation and widens its clinical applications. This short review delineates the biology of HIV-1-erived lentiviral vector, generation of integrase-deficient lentiviral vector, recent studies involving integrase-deficient lentiviral vectors, limitations, and prospects for neoteric clinical use.
Keywords: Cell Reprogramming; Cell death; Gene therapy; Immunization; Integrase-deficient lentiviral vector.
©2022 Yew et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures
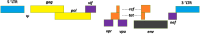

References
-
- Almarza D, Bussadori G, Navarro M, Mavilio F, Larcher F, Murillas R. Risk assessment in skin gene therapy: viral-cellular fusion transcripts generated by proviral transcriptional read-through in keratinocytes transduced with self-inactivating lentiviral vectors. Gene Therapy. 2011;18:674–681. doi: 10.1038/GT.2011.12. - DOI - PubMed
-
- Annoni A, Battaglia M, Follenzi A, Lombardo A, Sergi-Sergi L, Naldini L, Roncarolo M-G. The immune response to lentiviral-delivered transgene is modulated in vivo by transgene-expressing antigen-presenting cells but not by CD4+CD25+ regulatory T cells. Blood. 2007;110:1788–1796. doi: 10.1182/blood-2006-11-059873. - DOI - PubMed
-
- Ausubel LJ, Laderman KA, Shakeley M, Sharma A, Couture S, Lopez P, Knoblauch C, Anderson J, Derecho I, McMahon R, Hsu D, Couture L. 312. Large scale lentiviral vector production in a GMP facilty. Molecular Therapy. 2007;15(Suppl 1):S118. doi: 10.1016/s1525-0016(16)44518-1. - DOI
Further reading
-
- Anonymous. European Medicines AgencyGuideline on development and manufacture of lentiviral vectors. 2005
-
- Chen SH, Sun JM, Chen BM, Lin SC, Chang HF, Collins S, Chang D, Wu SF, Lu YC, Wang W, Chen TC, Kasahara N, Wang HE, Tai CK. Efficient prodrug activator gene therapy by retroviral replicating vectors prolongs survival in an immune-competent intracerebral glioma model. International Journal of Molecular Sciences. 2020;21(4):1433. doi: 10.3390/ijms21041433. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

