MicroRNA-22 suppresses NLRP3/CASP1 inflammasome pathway-mediated proinflammatory cytokine production by targeting the HIF-1α and NLRP3 in human dental pulp fibroblasts
- PMID: 35979583
- PMCID: PMC9805103
- DOI: 10.1111/iej.13814
MicroRNA-22 suppresses NLRP3/CASP1 inflammasome pathway-mediated proinflammatory cytokine production by targeting the HIF-1α and NLRP3 in human dental pulp fibroblasts
Abstract
Aim: To investigate the synergetic regulatory effect of miR-22 on HIF-1α and NLRP3, subsequently regulating the production of the NLRP3/CASP1 inflammasome pathway-mediated proinflammatory cytokines IL-1β and IL-18 in human dental pulp fibroblasts (HDPFs) during the progression of pulpitis.
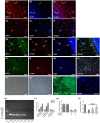
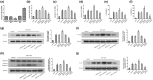
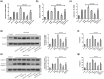
Methodology: Fluorescence in situ hybridization (FISH) and immunofluorescence (IF) were performed to determine the localization of miR-22-3p, NLRP3 and HIF-1α in human dental pulp tissues (HDPTs). The miR-22 mimics and inhibitor or plasmid of NLRP3 or HIF-1α were used to upregulate or downregulate miR-22 or NLRP3 or HIF-1α in HDPFs, respectively. Computational prediction via TargetScan 5.1 and a luciferase reporter assay were conducted to confirm target association. The mRNA and protein expression of HIF-1α, NLRP3, caspase-1, IL-1β and IL-18 were determined by qRT-PCR and western blotting, respectively. The release of IL-1β and IL-18 was analysed by ELISA. The significance of the differences between the experimental and control groups was determined by one-way analysis of variance, p < .05 indicated statistical significance.
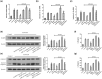
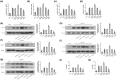
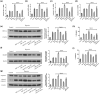
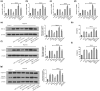
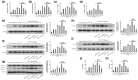
Results: A decrease in miR-22 and an increase in HIF-1α and NLRP3 in HDPTs occurred during the transformation of reversible pulpitis into irreversible pulpitis compared with that in the healthy pulp tissues (p < .05). In the normal HDPTs, miR-22-3p was extensively expressed in dental pulp cells. HIF-1α and NLRP3 were mainly expressed in the odontoblasts and vascular endothelial cells. Whereas in the inflamed HDPTs, the odontoblast layers were disrupted. HDPFs were positive for miR-22-3p, HIF-1α and NLRP3. Computational prediction via TargetScan 5.1 and luciferase reporter assays confirmed that both NLRP3 and HIF-1α were direct targets of miR-22 in HDPFs. The miR-22 inhibitor further promoted the activation of NLRP3/CASP1 inflammasome pathway induced by ATP plus LPS and hypoxia (p < .05). In contrast, the miR-22 mimic significantly inhibited the NLRP3/CASP1 inflammasome pathway activation induced by ATP plus LPS and hypoxia (p < .05).
Conclusion: MiR-22, as a synergetic negative regulator, is involved in controlling the secretion of proinflammatory cytokines mediated by the NLRP3/CASP1 inflammasome pathway by targeting NLRP3 and HIF-1α. These results provide a novel function and mechanism of miR-22-HIF-1α-NLRP3 signalling in the control of proinflammatory cytokine secretion, thus indicating a potential therapeutic strategy for future endodontic treatment.
Keywords: HIF-1α; IL-1β; NLRP3; human dental pulp fibroblasts; miR-22; pulpitis.
© 2022 The Authors. International Endodontic Journal published by John Wiley & Sons Ltd on behalf of British Endodontic Society.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures

References
-
- Chang, M.C. , Lin, L.D. , Zwei‐Ching Chang, J. , Huang, C.F. , Chuang, F.H. , Lee, J.J. et al. (2012) Regulation of vascular cell adhesion molecule‐1 in dental pulp cells by interleukin‐1beta: the role of prostanoids. Journal of Endodontis, 38, 774–779. - PubMed
-
- Chang, M.C. , Tsai, Y.L. , Chang, H.H. , Lee, S.Y. , Lee, M.S. , Chang, C.W. et al. (2016) IL‐1beta‐induced MCP‐1 expression and secretion of human dental pulp cells is related to TAK1, MEK/ERK, and PI3K/Akt signaling pathways. Archives of Oral Biology, 61, 16–22. - PubMed
MeSH terms
Substances
Grants and funding
- 2019KJXX-086/Innovative Talents Promotion Program-Youth Science and Technology Star Project
- 015216/Ling Yun Project-Eagle Programme from the Fourth Military Medical University
- 81700951/National Natural Science Foundation of China
- 81771095/National Natural Science Foundation of China
- 81803186/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Miscellaneous

