A mathematical model captures the role of adenyl cyclase Cyr1 and guanidine exchange factor Ira2 in creating a growth-to-hyphal bistable switch in Candida albicans
- PMID: 35979612
- PMCID: PMC9527597
- DOI: 10.1002/2211-5463.13470
A mathematical model captures the role of adenyl cyclase Cyr1 and guanidine exchange factor Ira2 in creating a growth-to-hyphal bistable switch in Candida albicans
Abstract
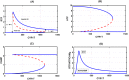
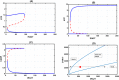
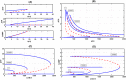
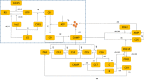
Recent biochemical experiments have indicated that in Candida albicans, a commensal fungal pathogen, the Ras signaling pathway plays a significant role in the yeast-to-hyphal transition; specifically, two enzymes in this pathway, Adenyl Cyclase Cyr1 and GTPase activating protein Ira2, facilitate this transition, in the presence of energy sensor ATP. However, the precise mechanism by which protein interactions between Ira2 and Cyr1 and the energy sensor ATP result in the yeast-to-hyphal transition and create a switch-like process are unknown. We propose a new set of biochemical reaction steps that captures all the essential interactions between Ira2, Cyr1, and ATP in the Ras pathway. With the help of chemical reaction network theory, we demonstrate that this set of biochemical reaction steps results in bistability. Further, bifurcation analysis of the differential equations based on this set of reaction steps supports the existence of a bistable switch, and this switch may act as a checkpoint mechanism for the promotion of growth-to-hyphal transition in C. albicans.
Keywords: Candida albicans; ODE; Ras signaling pathway; bistable; positive feedback; yeast-hyphal switching.
© 2022 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Comprehensive Interactome Analysis for the Sole Adenylyl Cyclase Cyr1 of Candida albicans.Microbiol Spectr. 2022 Dec 21;10(6):e0393422. doi: 10.1128/spectrum.03934-22. Epub 2022 Oct 31. Microbiol Spectr. 2022. PMID: 36314909 Free PMC article.
-
Molecular Docking Reveals Critical Residues in Candida albicans Cyr1 for Peptidoglycan Recognition and Hyphal Growth.ACS Infect Dis. 2023 Jul 14;9(7):1362-1371. doi: 10.1021/acsinfecdis.3c00115. Epub 2023 Jun 15. ACS Infect Dis. 2023. PMID: 37318518
-
Mitochondrial Activity and Cyr1 Are Key Regulators of Ras1 Activation of C. albicans Virulence Pathways.PLoS Pathog. 2015 Aug 28;11(8):e1005133. doi: 10.1371/journal.ppat.1005133. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26317337 Free PMC article.
-
Multiple roles and diverse regulation of the Ras/cAMP/protein kinase A pathway in Candida albicans.Mol Microbiol. 2019 Jan;111(1):6-16. doi: 10.1111/mmi.14148. Epub 2018 Nov 4. Mol Microbiol. 2019. PMID: 30299574 Review.
-
Candida albicans developmental regulation: adenylyl cyclase as a coincidence detector of parallel signals.Curr Opin Microbiol. 2011 Dec;14(6):682-6. doi: 10.1016/j.mib.2011.09.014. Epub 2011 Oct 17. Curr Opin Microbiol. 2011. PMID: 22014725 Review.
Cited by
-
Kinetic modelling reveals the presence of multistability in normal and stressful conditions in translational initiation mechanism.PLoS One. 2025 Mar 21;20(3):e0319280. doi: 10.1371/journal.pone.0319280. eCollection 2025. PLoS One. 2025. PMID: 40117264 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

