At the crux of maternal immune activation: Viruses, microglia, microbes, and IL-17A
- PMID: 35979731
- PMCID: PMC9804202
- DOI: 10.1111/imr.13125
At the crux of maternal immune activation: Viruses, microglia, microbes, and IL-17A
Abstract
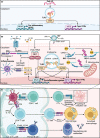
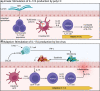
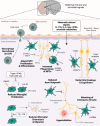
Inflammation during prenatal development can be detrimental to neurodevelopmental processes, increasing the risk of neuropsychiatric disorders. Prenatal exposure to maternal viral infection during pregnancy is a leading environmental risk factor for manifestation of these disorders. Preclinical animal models of maternal immune activation (MIA), established to investigate this link, have revealed common immune and microbial signaling pathways that link mother and fetus and set the tone for prenatal neurodevelopment. In particular, maternal intestinal T helper 17 cells, educated by endogenous microbes, appear to be key drivers of effector IL-17A signals capable of reaching the fetal brain and causing neuropathologies. Fetal microglial cells are particularly sensitive to maternally derived inflammatory and microbial signals, and they shift their functional phenotype in response to MIA. Resulting cortical malformations and miswired interneuron circuits cause aberrant offspring behaviors that recapitulate core symptoms of human neurodevelopmental disorders. Still, the popular use of "sterile" immunostimulants to initiate MIA has limited translation to the clinic, as these stimulants fail to capture biologically relevant innate and adaptive inflammatory sequelae induced by live pathogen infection. Thus, there is a need for more translatable MIA models, with a focus on relevant pathogens like seasonal influenza viruses.
Keywords: TH17 cells; influenza virus; maternal immune activation; microbial signaling; microglia; neurodevelopment.
© 2022 The Authors. Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Influenza A virus during pregnancy disrupts maternal intestinal immunity and fetal cortical development in a dose- and time-dependent manner.Mol Psychiatry. 2025 Jan;30(1):13-28. doi: 10.1038/s41380-024-02648-9. Epub 2024 Jul 3. Mol Psychiatry. 2025. PMID: 38961232 Free PMC article.
-
The Outcomes of Maternal Immune Activation Induced with the Viral Mimetic Poly I:C on Microglia in Exposed Rodent Offspring.Dev Neurosci. 2023;45(4):191-209. doi: 10.1159/000530185. Epub 2023 Mar 21. Dev Neurosci. 2023. PMID: 36944325 Review.
-
Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring.Nature. 2017 Sep 28;549(7673):528-532. doi: 10.1038/nature23910. Epub 2017 Sep 13. Nature. 2017. PMID: 28902840 Free PMC article.
-
Maternal immune activation-induced PPARγ-dependent dysfunction of microglia associated with neurogenic impairment and aberrant postnatal behaviors in offspring.Neurobiol Dis. 2019 May;125:1-13. doi: 10.1016/j.nbd.2019.01.005. Epub 2019 Jan 17. Neurobiol Dis. 2019. PMID: 30659984
-
Microglia, the missing link in maternal immune activation and fetal neurodevelopment; and a possible link in preeclampsia and disturbed neurodevelopment?J Reprod Immunol. 2018 Apr;126:18-22. doi: 10.1016/j.jri.2018.01.004. Epub 2018 Jan 31. J Reprod Immunol. 2018. PMID: 29421625 Review.
Cited by
-
An introduction to neuroimmunology.Immunol Rev. 2022 Oct;311(1):5-8. doi: 10.1111/imr.13133. Epub 2022 Aug 30. Immunol Rev. 2022. PMID: 36039857 Free PMC article. No abstract available.
-
Behavioral Test Scores Could Be Linked to the Protein Expression Values of p62 and GLAST in the Brains of Mice with Neuropsychiatric Disorder-Related Behaviors.Biology (Basel). 2024 Dec 11;13(12):1039. doi: 10.3390/biology13121039. Biology (Basel). 2024. PMID: 39765706 Free PMC article.
-
Interleukin-17A stimulation induces alterations in Microglial microRNA expression profiles.Pediatr Res. 2024 Jan;95(1):167-173. doi: 10.1038/s41390-023-02825-6. Epub 2023 Sep 27. Pediatr Res. 2024. PMID: 37758861
-
Interaction of the pre- and postnatal environment in the maternal immune activation model.Discov Ment Health. 2023;3(1):15. doi: 10.1007/s44192-023-00042-5. Epub 2023 Aug 22. Discov Ment Health. 2023. PMID: 37622027 Free PMC article. Review.
-
Maternal immune activation during the lactational period alters offspring behavior, reproductive development, and immune function in mice.Horm Behav. 2025 Jul;173:105776. doi: 10.1016/j.yhbeh.2025.105776. Epub 2025 Jun 20. Horm Behav. 2025. PMID: 40543230 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

