Aggressive corticotroph tumors and carcinomas
- PMID: 35979732
- PMCID: PMC9542524
- DOI: 10.1111/jne.13169
Aggressive corticotroph tumors and carcinomas
Abstract
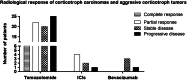
Pituitary tumors are generally benign, although in rare cases aggressive pituitary tumors (APTs) and carcinomas present important diagnostic and therapeutic challenges and are associated with a high mortality rate. Almost half of these APTs and carcinomas are corticotroph tumors, suggesting a specific prognosis. Clinical, pathological and molecular prognostic markers are limited and do not allow early management of these tumors. Temozolomide remains the first-line treatment once a diagnosis of aggressive pituitary tumor or carcinoma has been made. Novel alternative treatments exist, including immune checkpoint inhibitors, which can be used in the case of temozolomide treatment failure. The aim of this review is to present the clinical, pathological and molecular characteristics of aggressive corticotroph tumors and carcinomas, and to describe the results obtained with currently available treatments.
Keywords: Cushing's disease; aggressive pituitary tumor; corticotroph tumor; pituitary carcinoma; temozolomide.
© 2022 The Authors. Journal of Neuroendocrinology published by John Wiley & Sons Ltd on behalf of British Society for Neuroendocrinology.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

