Assessment of endocytic traffic and Ocrl function in the developing zebrafish neuroepithelium
- PMID: 35979861
- PMCID: PMC9592051
- DOI: 10.1242/jcs.260339
Assessment of endocytic traffic and Ocrl function in the developing zebrafish neuroepithelium
Abstract
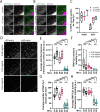
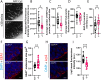
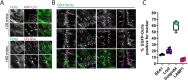
Endocytosis allows cells to internalise a wide range of molecules from their environment and to maintain their plasma membrane composition. It is vital during development and for maintenance of tissue homeostasis. The ability to visualise endocytosis in vivo requires suitable assays to monitor the process. Here, we describe imaging-based assays to visualise endocytosis in the neuroepithelium of living zebrafish embryos. Injection of fluorescent tracers into the brain ventricles followed by live imaging was used to study fluid-phase or receptor-mediated endocytosis, for which we used receptor-associated protein (RAP, encoded by Lrpap1) as a ligand for low-density lipoprotein receptor-related protein (LRP) receptors. Using dual-colour imaging combined with expression of endocytic markers, it is possible to track the progression of endocytosed tracers and to monitor trafficking dynamics. Using these assays, we reveal a role for the Lowe syndrome protein Ocrl in endocytic trafficking within the neuroepithelium. We also found that the RAP-binding receptor Lrp2 (encoded by lrp2a) appears to contribute only partially to neuroepithelial RAP endocytosis. Altogether, our results provide a basis to track endocytosis within the neuroepithelium in vivo and support a role for Ocrl in this process. This article has an associated First Person interview with the first author of the paper.
Keywords: Endocytosis; Lowe syndrome; Lrp2; Neuroepithelium; Ocrl; Zebrafish.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

