Pharmacokinetic behaviors of soft nanoparticulate formulations of chemotherapeutics
- PMID: 35979879
- PMCID: PMC9938089
- DOI: 10.1002/wnan.1846
Pharmacokinetic behaviors of soft nanoparticulate formulations of chemotherapeutics
Abstract
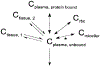
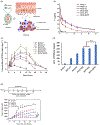
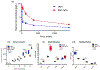
Chemotherapeutic treatment with conventional drug formulations pose numerous challenges, such as poor solubility, high cytotoxicity and serious off-target side effects, low bioavailability, and ultimately subtherapeutic tumoral concentration leading to poor therapeutic outcomes. In the field of Nanomedicine, advances in nanotechnology have been applied with great success to design and develop novel nanoparticle-based formulations for the treatment of various types of cancer. The approval of the first nanomedicine, Doxil® (liposomal doxorubicin) in 1995, paved the path for further development for various types of novel delivery platforms. Several different types of nanoparticles, especially organic (soft) nanoparticles (liposomes, polymeric micelles, and albumin-bound nanoparticles), have been developed and approved for several anticancer drugs. Nanoparticulate drug delivery platform have facilitated to overcome of these challenges and offered key advantages of improved bioavailability, higher intra-tumoral concentration of the drug, reduced toxicity, and improved efficacy. This review introduces various commonly used nanoparticulate systems in biomedical research and their pharmacokinetic (PK) attributes, then focuses on the various physicochemical and physiological factors affecting the in vivo disposition of chemotherapeutic agents encapsulated in nanoparticles in recent years. Further, it provides a review of the current landscape of soft nanoparticulate formulations for the two most widely investigated anticancer drugs, paclitaxel, and doxorubicin, that are either approved or under investigation. Formulation details, PK profiles, and therapeutic outcomes of these novel strategies have been discussed individually and in comparison, to traditional formulations. This article is categorized under: Nanotechnology Approaches to Biology > Cells at the Nanoscale Diagnostic Tools > In Vivo Nanodiagnostics and Imaging Therapeutic Approaches and Drug Discovery > Nanomedicine for Oncologic Disease.
Keywords: chemotherapeutics; doxorubicin; paclitaxel; pharmacokinetics; soft nanoparticles.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Figures
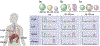


Similar articles
-
Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
-
The Clinical Translation of Organic Nanomaterials for Cancer Therapy: A Focus on Polymeric Nanoparticles, Micelles, Liposomes and Exosomes.Curr Med Chem. 2018;25(34):4224-4268. doi: 10.2174/0929867324666170830113755. Curr Med Chem. 2018. PMID: 28875844 Review.
-
Nanocarriers for anticancer drugs--new trends in nanomedicine.Curr Drug Metab. 2013 Jun;14(5):547-64. doi: 10.2174/1389200211314050005. Curr Drug Metab. 2013. PMID: 23687925 Review.
-
Liposomal therapies in oncology: does one size fit all?Cancer Chemother Pharmacol. 2018 Nov;82(5):741-755. doi: 10.1007/s00280-018-3668-7. Epub 2018 Aug 16. Cancer Chemother Pharmacol. 2018. PMID: 30116847 Review.
-
Liposomal doxorubicin and nab-paclitaxel: nanoparticle cancer chemotherapy in current clinical use.Methods Mol Biol. 2010;624:385-92. doi: 10.1007/978-1-60761-609-2_26. Methods Mol Biol. 2010. PMID: 20217610 Review.
Cited by
-
Advanced bioanalytical techniques for pharmacokinetic studies of nanocarrier drug delivery systems.J Pharm Anal. 2025 Jan;15(1):101070. doi: 10.1016/j.jpha.2024.101070. Epub 2024 Aug 14. J Pharm Anal. 2025. PMID: 39885973 Free PMC article. Review.
-
Accumulation of liposomes in metastatic tumor sites is not necessary for anti-cancer drug efficacy.J Transl Med. 2024 Jul 3;22(1):621. doi: 10.1186/s12967-024-05428-9. J Transl Med. 2024. PMID: 38961395 Free PMC article.
-
Nanotechnology-based approaches for targeted drug delivery for the treatment of respiratory tract infections.J Biol Methods. 2024 Oct 23;11(4):e99010032. doi: 10.14440/jbm.2024.0065. eCollection 2024. J Biol Methods. 2024. PMID: 39839091 Free PMC article. Review.
-
Paclitaxel in colon cancer management: from conventional chemotherapy to advanced nanocarrier delivery systems.Naunyn Schmiedebergs Arch Pharmacol. 2024 Dec;397(12):9449-9474. doi: 10.1007/s00210-024-03256-8. Epub 2024 Jul 11. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38990305 Review.
-
Trends in research on nanomedicine in urologic cancer: a bibliometric and visualized analysis.Discov Oncol. 2024 Aug 23;15(1):366. doi: 10.1007/s12672-024-01249-w. Discov Oncol. 2024. PMID: 39179938 Free PMC article.
References
-
- Ahmad S, Idris RAM, Wan Hanaffi WN, Perumal K, Boer JC, Plebanski M, Jaafar J, Lim JK, & Mohamud R (2021). Cancer nanomedicine and immune system—Interactions and challenges. Frontiers in Nanotechnology, 3. 10.3389/fnano.2021.681305 - DOI
-
- Alfayez M, Kantarjian H, Kadia T, Ravandi-Kashani F, & Daver N (2020). CPX-351 (vyxeos) in AML. Leukemia & Lymphoma, 61(2), 288–297. - PubMed
-
- Al-Jamal WT, Al-Ahmady ZS, & Kostarelos K (2012). Pharmacokinetics & tissue distribution of temperature-sensitive liposomal doxorubicin in tumor-bearing mice triggered with mild hyperthermia. Biomaterials, 33(18), 4608–4617. - PubMed
-
- Aronson JK (2016). Cremophor. In Aronson JK (Ed.), Meyler’s side effects of drugs (16th ed., p. 763). Elsevier.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

