Humoral response to heterologous prime-booster vaccination in heart transplant recipients aged 18-70 years primed with a viral vector SARS-CoV-2 vaccine
- PMID: 35980217
- PMCID: PMC9539221
- DOI: 10.1111/tid.13935
Humoral response to heterologous prime-booster vaccination in heart transplant recipients aged 18-70 years primed with a viral vector SARS-CoV-2 vaccine
Abstract
Solid organ transplant recipients have demonstrated a blunted immune response to standard 2-dose vaccination against SARS-CoV-2. This study sought to determine the humoral response to heterologous booster vaccination (viral vector vaccine dose 1 and 2 + mRNA booster). Heart transplant recipients, aged 18 to 70 years of age who initially received two doses of the viral vector ChAdOx1 nCoV-19 vaccine followed by a BNT162b2 mRNA booster were recruited. A detectable antibody response in the absence of prior SARS-CoV-2 was the primary outcome measured. This was defined as an anti-spike titre of ≥0.8 U/mL on the Elecsys anti-SARS-CoV-2 S immunoassay. A total of 80 heart transplant patients (mean age 49 ± 13 years, 28% female) were included. Blood samples were drawn at a median of 30 (IQR 28-33) days after the BNT162b2 mRNA booster. The frequency of a detectable antibody response increased from 37.5% (n = 30) after dose 2 to 56% (n = 45) post dose 3 (p < 0.001). A non-detectable antibody response was significantly more common in recipients with a shorter time interval from transplantation (p < 0.001), lower likelihood of cardiac allograft vasculopathy (p = 0.003) and in those prescribed a triple versus dual immunosuppressant regime (p = 0.009) and a tacrolimus versus cyclosporine basedregimen (p = 0.007). Despite heterologous prime-booster vaccination 44% of this vulnerable population ultimately continue to have no detectable antibodies.
Keywords: SARS-CoV-2; heart transplant; heterologous prime-booster vaccination; humoral response.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to disclose.
Figures
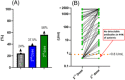
 frequency represents more than one person. Dotted line = threshold for detectable antibody response to SARS‐C0V‐2 vaccination (≥0.8 U/ml)
frequency represents more than one person. Dotted line = threshold for detectable antibody response to SARS‐C0V‐2 vaccination (≥0.8 U/ml)Similar articles
-
Humoral response to SARS-CoV-2 adenovirus vector vaccination (ChAdOx1 nCoV-19 [AZD1222]) in heart transplant recipients aged 18 to 70 years of age.J Heart Lung Transplant. 2022 Apr;41(4):492-500. doi: 10.1016/j.healun.2022.01.005. Epub 2022 Jan 10. J Heart Lung Transplant. 2022. PMID: 35090809 Free PMC article.
-
Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients.Clin Res Cardiol. 2021 Aug;110(8):1142-1149. doi: 10.1007/s00392-021-01880-5. Epub 2021 Jul 9. Clin Res Cardiol. 2021. PMID: 34241676 Free PMC article.
-
Humoral immune response after different SARS-CoV-2 vaccination regimens.BMC Med. 2022 Jan 21;20(1):31. doi: 10.1186/s12916-021-02231-x. BMC Med. 2022. PMID: 35057798 Free PMC article.
-
MOG encephalomyelitis after vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2): case report and comprehensive review of the literature.J Neurol. 2022 Oct;269(10):5198-5212. doi: 10.1007/s00415-022-11194-9. Epub 2022 Jun 23. J Neurol. 2022. PMID: 35737110 Free PMC article. Review.
-
SARS-CoV-2 vaccination in solid-organ transplant recipients: What the clinician needs to know.Transpl Int. 2021 Oct;34(10):1776-1788. doi: 10.1111/tri.14029. Epub 2021 Sep 20. Transpl Int. 2021. PMID: 34450686 Free PMC article. Review.
Cited by
-
Measures to Increase Immunogenicity of SARS-CoV-2 Vaccines in Solid Organ Transplant Recipients: A Narrative Review.Vaccines (Basel). 2023 Nov 25;11(12):1755. doi: 10.3390/vaccines11121755. Vaccines (Basel). 2023. PMID: 38140160 Free PMC article. Review.
References
-
- Itzhaki Ben Zadok O, Shaul AA, Ben‐Avraham B, et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients ‐ a prospective cohort study. Eur J Heart Fail. 2021;23(9):1555‐1559. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

