Long-term outcomes of osilodrostat in Cushing's disease: LINC 3 study extension
- PMID: 35980235
- PMCID: PMC9513654
- DOI: 10.1530/EJE-22-0317
Long-term outcomes of osilodrostat in Cushing's disease: LINC 3 study extension
Abstract
Objective: To investigate the long-term efficacy and tolerability of osilodrostat, a potent oral 11β-hydroxylase inhibitor, for treating Cushing's disease (CD).
Design/methods: A total of 137 adults with CD and mean 24-h urinary free cortisol (mUFC) > 1.5 × upper limit of normal (ULN) received osilodrostat (starting dose 2 mg bid; maximum 30 mg bid) during the prospective, Phase III, 48-week LINC 3 (NCT02180217) core study. Patients benefiting from osilodrostat at week 48 could enter the optional extension (ending when all patients had received ≥ 72 weeks of treatment or discontinued). Efficacy and safety were assessed for all enrolled patients from the core study baseline.
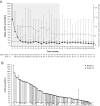
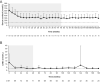
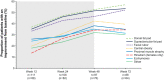
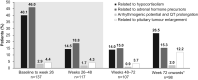
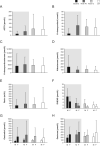
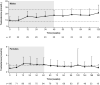
Results: Median osilodrostat exposure from the core study baseline to study end was 130 weeks (range 1-245) and median average dose was 7.4 mg/day (range 0.8-46.6). The reduction in mean mUFC achieved during the core was maintained during the extension and remained ≤ ULN. Of 106 patients, 86 (81%) patients who entered the extension had mUFC ≤ ULN at week 72. Improvements in cardiovascular/metabolic-related parameters, physical manifestations of hypercortisolism (fat pads, central obesity, rubor, striae, and hirsutism in females), and quality of life in the core study were also maintained or improved further during the extension. No new safety signals were reported; 15/137 (10.9%) and 12/106 (11.3%) patients discontinued for adverse events during the core and extension, respectively. Mean testosterone in females decreased towards baseline levels during the extension.
Conclusions: Data from this large, multicentre trial show that long-term treatment with osilodrostat sustains cortisol normalisation alongside clinical benefits in most patients with CD and is well tolerated.
Figures

Similar articles
-
Long-term efficacy and safety of osilodrostat in Cushing's disease: final results from a Phase II study with an optional extension phase (LINC 2).Pituitary. 2022 Dec;25(6):959-970. doi: 10.1007/s11102-022-01280-6. Epub 2022 Oct 11. Pituitary. 2022. PMID: 36219274 Free PMC article. Clinical Trial.
-
Improvement in clinical features of hypercortisolism during osilodrostat treatment: findings from the Phase III LINC 3 trial in Cushing's disease.J Endocrinol Invest. 2024 Oct;47(10):2437-2448. doi: 10.1007/s40618-024-02359-6. Epub 2024 May 2. J Endocrinol Invest. 2024. PMID: 38696122 Free PMC article. Clinical Trial.
-
Efficacy and safety of osilodrostat in patients with Cushing's disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase.Lancet Diabetes Endocrinol. 2020 Sep;8(9):748-761. doi: 10.1016/S2213-8587(20)30240-0. Epub 2020 Jul 27. Lancet Diabetes Endocrinol. 2020. PMID: 32730798 Clinical Trial.
-
Osilodrostat for the treatment of Cushing's disease.Expert Opin Pharmacother. 2021 Jun;22(9):1099-1106. doi: 10.1080/14656566.2021.1897106. Epub 2021 Mar 11. Expert Opin Pharmacother. 2021. PMID: 33703978 Review.
-
Osilodrostat - an emerging drug for the medical management of Cushing's disease.Endokrynol Pol. 2022;73(2):371-374. doi: 10.5603/EP.a2022.0009. Epub 2022 Apr 5. Endokrynol Pol. 2022. PMID: 35381096 Review.
Cited by
-
Safety assessment of Osilodrostat: The adverse event analysis based on FAERS database by means of disproportionality analysis.PLoS One. 2025 Aug 7;20(8):e0329088. doi: 10.1371/journal.pone.0329088. eCollection 2025. PLoS One. 2025. PMID: 40773444 Free PMC article.
-
Prolonged Adrenal Insufficiency After Osilodrostat Exposure With Eventual Recovery of Adrenal Function.JCEM Case Rep. 2024 Jun 3;2(6):luae088. doi: 10.1210/jcemcr/luae088. eCollection 2024 Jun. JCEM Case Rep. 2024. PMID: 38832004 Free PMC article.
-
Long-term efficacy and safety of osilodrostat in Cushing's disease: final results from a Phase II study with an optional extension phase (LINC 2).Pituitary. 2022 Dec;25(6):959-970. doi: 10.1007/s11102-022-01280-6. Epub 2022 Oct 11. Pituitary. 2022. PMID: 36219274 Free PMC article. Clinical Trial.
-
Selectivity of osilodrostat as an inhibitor of human steroidogenic cytochromes P450.J Steroid Biochem Mol Biol. 2023 Jul;231:106316. doi: 10.1016/j.jsbmb.2023.106316. Epub 2023 Apr 23. J Steroid Biochem Mol Biol. 2023. PMID: 37098354 Free PMC article.
-
Osilodrostat treatment in patients with Cushing's disease of Asian or non-Asian origin: a pooled analysis of two Phase III randomized trials (LINC 3 and LINC 4).Endocr J. 2024 Dec 2;71(12):1103-1123. doi: 10.1507/endocrj.EJ24-0153. Epub 2024 Aug 23. Endocr J. 2024. PMID: 39183039 Free PMC article. Clinical Trial.

