MiR-375 mitigates retinal angiogenesis by depressing the JAK2/STAT3 pathway
- PMID: 35980290
- PMCID: PMC9467412
- DOI: 10.18632/aging.204232
MiR-375 mitigates retinal angiogenesis by depressing the JAK2/STAT3 pathway
Abstract
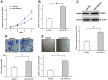
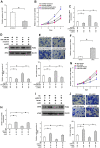
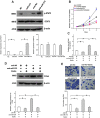
Aberrant neovascularization in the retina is an important threat to vision and closely related to several retinal diseases, such as wet form of age-related macular degeneration, diabetic retinopathy, and retinopathy of prematurity. However, the pathogenesis remains largely unknown. MicroRNAs (miRNAs) have been demonstrated to play critical regulatory roles in angiogenesis. Therefore, we aimed to identify the key miRNAs that regulate retinal neovascularization and elucidate the potential underlying mechanisms. In the present study, we performed RNA sequencing of microRNAs in the retina and found that miR-375 was significantly downregulated in the retina of oxygen-induced retinopathy mice. In retinal microvascular endothelial cells (RMECs), overexpression of miR-375 inhibited cell proliferation and angiogenesis. Conversely, inhibition of miR-375 had the opposite effects. Moreover, our results showed that miR-375 negatively regulated the protein expression of JAK2 by inhibiting its translation. The promoting effects of anti-miR-375 on cell proliferation and angiogenesis were attenuated by an inhibitor of STAT3. These results indicate that miR-375 mitigates cell proliferation and angiogenesis, at least in part, through the JAK2/STAT3 pathway in RMECs, which implies an important underlying mechanism of retinal angiogenesis and provides potential therapeutic targets for retinal microangiopathy.
Keywords: JAK2; angiogenesis; miR-375; proliferation; retina.
Conflict of interest statement
Figures



Similar articles
-
MicroRNA-29b-3p inhibits cell proliferation and angiogenesis by targeting VEGFA and PDGFB in retinal microvascular endothelial cells.Mol Vis. 2020 Feb 24;26:64-75. eCollection 2020. Mol Vis. 2020. PMID: 32165827 Free PMC article.
-
C-CBL is required for inhibition of angiogenesis through modulating JAK2/STAT3 activity in ROP development.Biomed Pharmacother. 2020 Dec;132:110856. doi: 10.1016/j.biopha.2020.110856. Epub 2020 Oct 28. Biomed Pharmacother. 2020. PMID: 33125970 Free PMC article.
-
MicroRNA-384-3p inhibits retinal neovascularization through targeting hexokinase 2 in mice with diabetic retinopathy.J Cell Physiol. 2018 Jan;234(1):721-730. doi: 10.1002/jcp.26871. Epub 2018 Sep 7. J Cell Physiol. 2018. PMID: 30191948
-
Molecular Mediators and Regulators of Retinal Angiogenesis.Semin Ophthalmol. 2023 Feb;38(2):124-133. doi: 10.1080/08820538.2022.2152706. Epub 2022 Dec 19. Semin Ophthalmol. 2023. PMID: 36536520 Review.
-
The Role of MiR-181 Family Members in Endothelial Cell Dysfunction and Tumor Angiogenesis.Cells. 2022 May 18;11(10):1670. doi: 10.3390/cells11101670. Cells. 2022. PMID: 35626707 Free PMC article. Review.
Cited by
-
The potential of vascular normalization for sensitization to radiotherapy.Heliyon. 2024 Jun 8;10(12):e32598. doi: 10.1016/j.heliyon.2024.e32598. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38952362 Free PMC article. Review.
-
Unveiling the role of CaMKII in retinal degeneration: from biological mechanism to therapeutic strategies.Cell Biosci. 2024 May 9;14(1):59. doi: 10.1186/s13578-024-01236-2. Cell Biosci. 2024. PMID: 38725013 Free PMC article. Review.
-
Correlation analysis of serum miRNA expression levels with the degree of macular edema in patients with retinal vein occlusion and its clinical implications.Front Neurol. 2025 Jul 22;16:1603790. doi: 10.3389/fneur.2025.1603790. eCollection 2025. Front Neurol. 2025. PMID: 40765619 Free PMC article.
-
The role of non-coding RNA in the diagnosis and treatment of Helicobacter pylori-related gastric cancer, with a focus on inflammation and immune response.Front Med (Lausanne). 2022 Oct 13;9:1009021. doi: 10.3389/fmed.2022.1009021. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36314013 Free PMC article. Review.
-
MicroRNA-375 modulates neutrophil chemotaxis via targeting Cathepsin B in zebrafish.Fish Shellfish Immunol. 2024 Nov;154:109933. doi: 10.1016/j.fsi.2024.109933. Epub 2024 Sep 28. Fish Shellfish Immunol. 2024. PMID: 39343064
References
-
- Mahdi A, Darvishi B, Majidzadeh-A K, Salehi M, Farahmand L. Challenges facing antiangiogenesis therapy: The significant role of hypoxia-inducible factor and MET in development of resistance to anti-vascular endothelial growth factor-targeted therapies. J Cell Physiol. 2019; 234:5655–63. 10.1002/jcp.27414 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

