Clinical and experimental treatment of type 1 diabetes
- PMID: 35980300
- PMCID: PMC9750829
- DOI: 10.1093/cei/uxac077
Clinical and experimental treatment of type 1 diabetes
Abstract
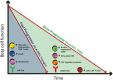
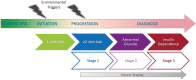
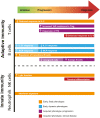
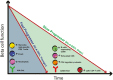
Type 1 diabetes (T1D) is an autoimmune disease resulting in the destruction of the insulin-producing pancreatic beta cells. Disease progression occurs along a trajectory from genetic risk, the development of islet autoantibodies, and autoreactive T cells ultimately progressing to clinical disease. Natural history studies and mechanistic studies linked to clinical trials have provided insight into the role of the immune system in disease pathogenesis. Here, we review our current understanding of the underlying etiology of T1D, focusing on the immune cell types that have been implicated in progression from pre-symptomatic T1D to clinical diagnosis and established disease. This knowledge has been foundational for the development of immunotherapies aimed at the prevention and treatment of T1D.
Keywords: autoantibodies; autoimmunity; cellular immunology; diabetes; immunotherapy.
© The Author(s) 2022. Published by Oxford University Press on behalf of the British Society for Immunology.
Conflict of interest statement
S.A.L. is a consultant for Sonoma Biotherapeutics, a member of the Type 1 Diabetes TrialNet Mechanistic Assay Group, a member of the Immune Tolerance Network Mechanistic Assay Group, and has received past research support from Caladrius Biosciences, Sonoma Biotherapeutics, and Janssen. J.H.B. is a Scientific Co-Founder and Scientific Advisory Board member of GentiBio, a consultant for Bristol-Myers Squibb and Hotspot Therapeutics, and has past and current research projects sponsored by Amgen, Bristol-Myers Squib, Janssen, Novo Nordisk, and Pfizer. She is a member of the Type 1 Diabetes TrialNet Study Group, a partner of the Allen Institute for Immunology, and a member of the Scientific Advisory Boards for the La Jolla Institute for Allergy and Immunology and BMS Immunology.
Figures
Similar articles
-
Immunotherapy trials for type 1 diabetes: the contribution of George Eisenbarth.Diabetes Technol Ther. 2013 Jun;15 Suppl 2(Suppl 2):S2-13-S2-20. doi: 10.1089/dia.2013.0107. Diabetes Technol Ther. 2013. PMID: 23786294 Free PMC article.
-
Protective role of anti-idiotypic antibodies in autoimmunity--lessons for type 1 diabetes.Autoimmunity. 2012 Jun;45(4):320-31. doi: 10.3109/08916934.2012.659299. Epub 2012 Feb 23. Autoimmunity. 2012. PMID: 22288464 Free PMC article. Review.
-
Lessons and gaps in the prediction and prevention of type 1 diabetes.Pharmacol Res. 2023 Jul;193:106792. doi: 10.1016/j.phrs.2023.106792. Epub 2023 May 16. Pharmacol Res. 2023. PMID: 37201589 Review.
-
The case for an autoimmune aetiology of type 1 diabetes.Clin Exp Immunol. 2016 Jan;183(1):8-15. doi: 10.1111/cei.12699. Epub 2015 Oct 21. Clin Exp Immunol. 2016. PMID: 26313217 Free PMC article. Review.
-
Lessons from type 1 diabetes for understanding natural history and prevention of autoimmune disease.Rheum Dis Clin North Am. 2014 Nov;40(4):797-811. doi: 10.1016/j.rdc.2014.07.008. Epub 2014 Sep 2. Rheum Dis Clin North Am. 2014. PMID: 25437293 Free PMC article. Review.
Cited by
-
Antigen-specific immunotherapies for autoimmune disease.Nat Rev Rheumatol. 2025 Feb;21(2):88-97. doi: 10.1038/s41584-024-01201-w. Epub 2024 Dec 16. Nat Rev Rheumatol. 2025. PMID: 39681709 Review.
-
Immune-related adverse events after immune check point inhibitors: Understanding the intersection with autoimmunity.Immunol Rev. 2023 Sep;318(1):81-88. doi: 10.1111/imr.13247. Epub 2023 Jul 26. Immunol Rev. 2023. PMID: 37493210 Free PMC article. Review.
-
Clinical and Experimental Immunology: highlights from 2022.Clin Exp Immunol. 2023 Apr 7;212(1):11-13. doi: 10.1093/cei/uxad018. Clin Exp Immunol. 2023. PMID: 36805630 Free PMC article. No abstract available.
-
Introducing our new series: Clinical and Experimental Treatment of….Clin Exp Immunol. 2022 Dec 15;210(2):104. doi: 10.1093/cei/uxac100. Clin Exp Immunol. 2022. PMID: 36355570 Free PMC article. No abstract available.
-
Beyond inflammation: the multifaceted therapeutic potential of targeting the CXCL8-CXCR1/2 axis in type 1 diabetes.Front Immunol. 2025 Jul 11;16:1576371. doi: 10.3389/fimmu.2025.1576371. eCollection 2025. Front Immunol. 2025. PMID: 40718478 Free PMC article. Review.
References
-
- World Health Organization. Diabetes, 2022. https://www.who.int/health-topics/diabetes#tab=tab_1
-
- Steck AK, Vehik K, Bonifacio E, Lernmark A, Ziegler AG, Hagopian WA, et al. . Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 2015, 38, 808–13. doi:10.2337/dc14-2426. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

