Halogen Atom Participation in Guiding the Stereochemical Outcomes of Acetal Substitution Reactions
- PMID: 35980341
- PMCID: PMC9561118
- DOI: 10.1002/anie.202209401
Halogen Atom Participation in Guiding the Stereochemical Outcomes of Acetal Substitution Reactions
Abstract
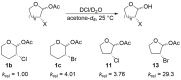
Acetal substitution reactions of α-halogenated five- and six-membered rings can be highly stereoselective. Erosion of stereoselectivity occurs as nucleophilicity increases, which is consistent with additions to a halogen-stabilized oxocarbenium ion, not a three-membered-ring halonium ion. Computational investigations confirmed that the open-form oxocarbenium ions are the reactive intermediates involved. Kinetic studies suggest that hyperconjugative effects and through-space electrostatic interactions can both contribute to the stabilization of halogen-substituted oxocarbenium ions.
Keywords: Diastereoselectivity; Glycosylation; Halonium Ion; Hyperconjugation; Oxocarbenium Ion.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Influence of Halogen Atoms and the Reactivity of Nucleophiles on Reactions of Tetrahydropyran and Tetrahydrofuran Acetals with C-Nucleophiles: Hyperconjugation and Inductive Effects.J Org Chem. 2025 Jul 18;90(28):9673-9687. doi: 10.1021/acs.joc.4c03128. Epub 2025 Jul 3. J Org Chem. 2025. PMID: 40607745 Free PMC article.
-
Effect of conformational rigidity on the stereoselectivity of nucleophilic additions to five-membered ring bicyclic oxocarbenium ion intermediates.Org Biomol Chem. 2014 Sep 28;12(36):7083-91. doi: 10.1039/c4ob01251h. Org Biomol Chem. 2014. PMID: 25087588 Free PMC article.
-
Diastereoselective nucleophilic substitution reactions of oxasilacyclopentane acetals: application of the "inside attack" model for reactions of five-membered ring oxocarbenium ions.J Org Chem. 2002 Apr 5;67(7):2056-64. doi: 10.1021/jo010823m. J Org Chem. 2002. PMID: 11925209
-
The Experimental Evidence in Support of Glycosylation Mechanisms at the SN1-SN2 Interface.Chem Rev. 2018 Sep 12;118(17):8242-8284. doi: 10.1021/acs.chemrev.8b00083. Epub 2018 May 30. Chem Rev. 2018. PMID: 29846062 Free PMC article. Review.
-
Glycosyl Oxocarbenium Ions: Structure, Conformation, Reactivity, and Interactions.Acc Chem Res. 2021 Jun 1;54(11):2552-2564. doi: 10.1021/acs.accounts.1c00021. Epub 2021 Apr 30. Acc Chem Res. 2021. PMID: 33930267 Free PMC article. Review.
Cited by
-
Influence of Halogen Atoms and the Reactivity of Nucleophiles on Reactions of Tetrahydropyran and Tetrahydrofuran Acetals with C-Nucleophiles: Hyperconjugation and Inductive Effects.J Org Chem. 2025 Jul 18;90(28):9673-9687. doi: 10.1021/acs.joc.4c03128. Epub 2025 Jul 3. J Org Chem. 2025. PMID: 40607745 Free PMC article.
-
Neighboring-Group Participation by a Less Electron-Donating, Participating C-2-Ester Ensures Higher 1,2-trans Stereoselectivity in Nucleophilic Substitution Reactions of Furanosyl Acetals.J Org Chem. 2025 Jan 31;90(4):1585-1596. doi: 10.1021/acs.joc.4c02612. Epub 2025 Jan 15. J Org Chem. 2025. PMID: 39813125 Free PMC article.
-
Acetal Substitution Reactions: Stereoelectronic Effects, Conformational Analysis, Reactivity vs. Selectivity, and Neighboring-Group Participation.Synlett. 2024 Sep;35(15):1763-1787. doi: 10.1055/s-0042-1751541. Epub 2024 Jan 16. Synlett. 2024. PMID: 39502501 Free PMC article.
-
Anomeric Triflates versus Dioxanium Ions: Different Product-Forming Intermediates from 3-Acyl Benzylidene Mannosyl and Glucosyl Donors.J Org Chem. 2024 Feb 2;89(3):1618-1625. doi: 10.1021/acs.joc.3c02262. Epub 2024 Jan 18. J Org Chem. 2024. PMID: 38235652 Free PMC article.
-
Neighboring-Group Participation by C-2 Acyloxy Groups: Influence of the Nucleophile and Acyl Group on the Stereochemical Outcome of Acetal Substitution Reactions.Chemistry. 2023 Oct 13;29(57):e202301894. doi: 10.1002/chem.202301894. Epub 2023 Sep 11. Chemistry. 2023. PMID: 37410662 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

