Cost-effectiveness analysis of gene-based therapies for patients with spinal muscular atrophy type I in Australia
- PMID: 35980467
- PMCID: PMC9618547
- DOI: 10.1007/s00415-022-11319-0
Cost-effectiveness analysis of gene-based therapies for patients with spinal muscular atrophy type I in Australia
Abstract
Introduction: Spinal muscular atrophy (SMA) is an inherited neuromuscular disorder and regarded as one of the most frequent genetic causes of infant mortality. The aim of this study is to develop a cost-effectiveness analysis of AVXS-101 (Onasemnogene Abeparvovec/Zolgensma®) and nusinersen (Spinraza®) for SMA to inform decision-making on reimbursement policies in Australia.
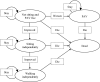
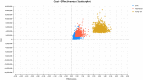
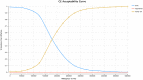
Methods: A Markov model was developed with five health states to evaluate the costs and effects for patients with SMA Type I from a healthcare system perspective over a time-horizon of 100 years. The model parameters were based on clinical trials, parametric distributions, published literature, and Australian registries. One-way and probabilistic sensitivity analysis were performed to appraise the uncertainties of the parameters in the model. A threshold analysis was conducted to estimate the cost of AVXS-101 of being cost-effective.
Results: The incremental cost-effectiveness ratio (ICER) of AVXS-101 was $1,808,471 per quality-adjusted life year (QALY) and that of nusinersen was $2,772,798 per QALY, compared to standard of care, respectively. The ICER of AVXS-101 was $1,238,288 per QALY compared to nusinersen. The key drivers influencing on ICERs were costs of using treatments and utility values of sitting and walking independently.
Conclusion: Both nusinersen and AVXS-101 resulted in health benefits, but they were not cost-effective with a commonly used willingness-to-pay (WTP) threshold of $50,000 per QALY. Developing high-quality clinical data and exploring appropriate WTP thresholds are critical for decision-making on reimbursement policies in the treatment of rare diseases.
Keywords: Cost-effectiveness analysis; Genetic therapy; Nusinersen; Onasemnogene abeparvovec; Rare disease; Spinal muscular atrophy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Early Cost-Effectiveness of Onasemnogene Abeparvovec-xioi (Zolgensma) and Nusinersen (Spinraza) Treatment for Spinal Muscular Atrophy I in The Netherlands With Relapse Scenarios.Value Health. 2021 Jun;24(6):759-769. doi: 10.1016/j.jval.2020.09.021. Epub 2021 Mar 31. Value Health. 2021. PMID: 34119073
-
An updated cost-utility model for onasemnogene abeparvovec (Zolgensma®) in spinal muscular atrophy type 1 patients and comparison with evaluation by the Institute for Clinical and Effectiveness Review (ICER).J Mark Access Health Policy. 2021 Feb 28;9(1):1889841. doi: 10.1080/20016689.2021.1889841. J Mark Access Health Policy. 2021. PMID: 33708361 Free PMC article.
-
Cost-Effectiveness of Onasemnogene Abeparvovec Compared With Nusinersen and Risdiplam in Patients With Spinal Muscular Atrophy Type 1 in Brazil: Custo-Efetividade do Onasemnogeno Abeparvoveque (AVXS-101) em Comparação ao Nusinersena e Risdiplam em Pacientes com Atrofia Muscular Espinhal Tipo 1 no Brasil.Value Health Reg Issues. 2024 Mar;40:108-117. doi: 10.1016/j.vhri.2023.11.004. Epub 2024 Jan 5. Value Health Reg Issues. 2024. PMID: 38181723
-
Gene Therapy for Spinal Muscular Atrophy (SMA): A Review of Current Challenges and Safety Considerations for Onasemnogene Abeparvovec (Zolgensma).Cureus. 2023 Mar 15;15(3):e36197. doi: 10.7759/cureus.36197. eCollection 2023 Mar. Cureus. 2023. PMID: 37065340 Free PMC article. Review.
-
Matching-adjusted indirect treatment comparison of onasemnogene abeparvovec and nusinersen for the treatment of symptomatic patients with spinal muscular atrophy type 1.Curr Med Res Opin. 2021 Oct;37(10):1719-1730. doi: 10.1080/03007995.2021.1947216. Epub 2021 Jul 20. Curr Med Res Opin. 2021. PMID: 34236007
Cited by
-
Antisense oligonucleotides and their applications in rare neurological diseases.Front Neurosci. 2024 Sep 23;18:1414658. doi: 10.3389/fnins.2024.1414658. eCollection 2024. Front Neurosci. 2024. PMID: 39376536 Free PMC article. Review.
-
Multiplex Real-Time PCR-Based Newborn Screening for Severe Primary Immunodeficiency and Spinal Muscular Atrophy in Osaka, Japan: Our Results after 3 Years.Genes (Basel). 2024 Feb 28;15(3):314. doi: 10.3390/genes15030314. Genes (Basel). 2024. PMID: 38540372 Free PMC article.
-
Current clinical applications of AAV-mediated gene therapy.Mol Ther. 2025 Jun 4;33(6):2479-2516. doi: 10.1016/j.ymthe.2025.04.045. Epub 2025 May 5. Mol Ther. 2025. PMID: 40329530 Review.
-
Cost-effectiveness of IVF with PGT-M/A to prevent transmission of spinal muscular atrophy in offspring of carrier couples.J Assist Reprod Genet. 2023 Apr;40(4):793-801. doi: 10.1007/s10815-023-02738-7. Epub 2023 Feb 9. J Assist Reprod Genet. 2023. PMID: 36757555 Free PMC article.
-
The Socio-Economic Burden of Spinal Muscular Atrophy: A Cost-of-Illness Study in Bulgaria.Healthcare (Basel). 2025 Feb 13;13(4):401. doi: 10.3390/healthcare13040401. Healthcare (Basel). 2025. PMID: 39997276 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical