Association of Predominantly Peripheral Lesions on Ultra-Widefield Imaging and the Risk of Diabetic Retinopathy Worsening Over Time
- PMID: 35980608
- PMCID: PMC9389433
- DOI: 10.1001/jamaophthalmol.2022.3131
Association of Predominantly Peripheral Lesions on Ultra-Widefield Imaging and the Risk of Diabetic Retinopathy Worsening Over Time
Erratum in
-
Addition of Nonauthor Collaborator Names for the DRCR Retina Network.JAMA Ophthalmol. 2023 Jan 1;141(1):104. doi: 10.1001/jamaophthalmol.2022.5280. JAMA Ophthalmol. 2023. PMID: 36394864 Free PMC article. No abstract available.
Abstract
Importance: Ultra-widefield (UWF) imaging improves the ability to identify peripheral diabetic retinopathy (DR) lesions compared with standard imaging. Whether detection of predominantly peripheral lesions (PPLs) better predicts rates of disease worsening over time is unknown.
Objective: To determine whether PPLs identified on UWF imaging are associated with increased disease worsening beyond the risk associated with baseline Early Treatment Diabetic Retinopathy Study (ETDRS) Diabetic Retinopathy Severity Scale (DRSS) score.
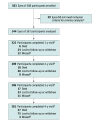
Design, setting, and participants: This cohort study was a prospective, multicenter, longitudinal observational study conducted at 37 US and Canadian sites with 388 participants enrolled between February and December 2015. At baseline and annually through 4 years, 200° UWF-color images were obtained and graded for DRSS at a reading center. Baseline UWF-color and UWF-fluorescein angiography (FA) images were evaluated for the presence of PPL. Data were analyzed from May 2020 to June 2022.
Interventions: Treatment of DR or diabetic macular edema was at investigator discretion.
Main outcomes and measures: Predominantly peripheral lesions were defined as DR lesions with a greater extent outside vs inside the 7 standard ETDRS fields. Primary outcome was disease worsening defined as worsening 2 steps or more on the DRSS or receipt of DR treatment. Analyses were adjusted for baseline DRSS score and correlation between 2 study eyes of the same participant.
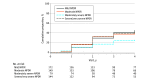
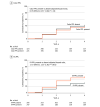
Results: Data for 544 study eyes with nonproliferative DR (NPDR) were analyzed (182 [50%] female participants; median age, 62 years; 68% White). The 4-year disease worsening rates were 45% for eyes with baseline mild NPDR, 40% for moderate NPDR, 26% for moderately severe NPDR, and 43% for severe NPDR. Disease worsening was not associated with color PPL at baseline (present vs absent: 38% vs 43%; HR, 0.78; 95% CI, 0.57-1.08; P = .13) but was associated with FA PPL at baseline (present vs absent: 50% vs 31%; HR, 1.72; 95% CI, 1.25-2.36; P < .001).
Conclusions and relevance: Although no association was identified with color PPL, presence of FA PPL was associated with greater risk of disease worsening over 4 years, independent of baseline DRSS score. These results suggest that use of UWF-FA to evaluate retinas peripheral to standard ETDRS fields may improve the ability to predict disease worsening in NPDR eyes. These findings support use of UWF-FA for future DR staging systems and clinical care to more accurately determine prognosis in NPDR eyes.
Conflict of interest statement
Figures
Comment on
-
Thinking Outside the Circle-The Potential Value of Ultra-Widefield Imaging.JAMA Ophthalmol. 2022 Oct 1;140(10):955-956. doi: 10.1001/jamaophthalmol.2022.3166. JAMA Ophthalmol. 2022. PMID: 35980651 No abstract available.
References
-
- Early Treatment Diabetic Retinopathy Study Research Group . Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology. 1991;98(5)(suppl):786-806. doi:10.1016/S0161-6420(13)38012-9 - DOI - PubMed
-
- Silva PS, Cavallerano JD, Sun JK, Noble J, Aiello LM, Aiello LP. Nonmydriatic ultrawide field retinal imaging compared with dilated standard 7-field 35-mm photography and retinal specialist examination for evaluation of diabetic retinopathy. Am J Ophthalmol. 2012;154(3):549-559.e2. doi:10.1016/j.ajo.2012.03.019 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

