Association of Ultra-Widefield Fluorescein Angiography-Identified Retinal Nonperfusion and the Risk of Diabetic Retinopathy Worsening Over Time
- PMID: 35980610
- PMCID: PMC9389436
- DOI: 10.1001/jamaophthalmol.2022.3130
Association of Ultra-Widefield Fluorescein Angiography-Identified Retinal Nonperfusion and the Risk of Diabetic Retinopathy Worsening Over Time
Erratum in
-
Addition of Nonauthor Collaborator Names for the DRCR Retina Network.JAMA Ophthalmol. 2023 Jan 1;141(1):104. doi: 10.1001/jamaophthalmol.2022.5277. JAMA Ophthalmol. 2023. PMID: 36394878 Free PMC article. No abstract available.
Abstract
Importance: Presence of predominantly peripheral diabetic retinopathy (DR) lesions on ultra-widefield fluorescein angiography (UWF-FA) was associated with greater risk of DR worsening or treatment over 4 years. Whether baseline retinal nonperfusion assessment is additionally predictive of DR disease worsening is unclear.
Objective: To assess whether the extent and location of retinal nonperfusion identified on UWF-FA are associated with worsening in Diabetic Retinopathy Severity Scale (DRSS) score or DR treatment over time.
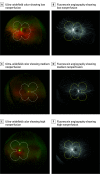
Design, setting, and participants: This cohort study was a prospective, multicenter, longitudinal observational study with data for 508 eyes with nonproliferative DR and gradable nonperfusion on UWF-FA at baseline. All images were graded at a centralized reading center; 200° ultra-widefield (UWF) color images were graded for DR at baseline and annually for 4 years. Baseline 200° UWF-FA images were graded for nonperfused area, nonperfusion index (NPI), and presence of predominantly peripheral lesions on UWF-FA (FA PPL).
Interventions: Treatment of DR or diabetic macular edema was at investigator discretion.
Main outcomes and measures: Association of baseline UWF-FA nonperfusion extent with disease worsening, defined as either 2 or more steps of DRSS worsening within Early Treatment Diabetic Retinopathy Study fields on UWF-color images or receipt of DR treatment.
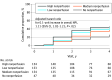
Results: After adjusting for baseline DRSS, the risk of disease worsening over 4 years was higher in eyes with greater overall NPI (hazard ratio [HR] for 0.1-unit increase, 1.11; 95% CI, 1.02-1.21; P = .02) and NPI within the posterior pole (HR for 0.1-unit increase, 1.35; 95% CI, 1.17-1.56; P < .001) and midperiphery (HR for 0.1-unit increase, 1.08; 95% CI, 1.00-1.16; P = .04). In a multivariable analysis adjusting for baseline DRSS score and baseline systemic risk factors, greater NPI (HR, 1.11; 95% CI, 1.02-1.22; P = .02) and presence of FA PPL (HR, 1.89; 95% CI, 1.35-2.65; P < .001) remained associated with disease worsening.
Conclusions and relevance: This 4-year longitudinal study has demonstrated that both greater baseline retinal nonperfusion and FA PPL on UWF-FA are associated with higher risk of disease worsening, even after adjusting for baseline DRSS score and known systemic risk. These associations between disease worsening and retinal nonperfusion and FA PPL support the increased use of UWF-FA to complement color fundus photography in future efforts for DR prognosis, clinical care, and research.
Conflict of interest statement
Figures
Comment on
-
Thinking Outside the Circle-The Potential Value of Ultra-Widefield Imaging.JAMA Ophthalmol. 2022 Oct 1;140(10):955-956. doi: 10.1001/jamaophthalmol.2022.3166. JAMA Ophthalmol. 2022. PMID: 35980651 No abstract available.
Similar articles
-
Association of Predominantly Peripheral Lesions on Ultra-Widefield Imaging and the Risk of Diabetic Retinopathy Worsening Over Time.JAMA Ophthalmol. 2022 Oct 1;140(10):946-954. doi: 10.1001/jamaophthalmol.2022.3131. JAMA Ophthalmol. 2022. PMID: 35980608 Free PMC article.
-
ASSESSMENT OF FLUORESCEIN ANGIOGRAPHY NONPERFUSION IN EYES WITH DIABETIC RETINOPATHY USING ULTRAWIDE FIELD RETINAL IMAGING.Retina. 2022 Jul 1;42(7):1302-1310. doi: 10.1097/IAE.0000000000003479. Retina. 2022. PMID: 35344528 Free PMC article.
-
Diabetic Retinopathy Severity and Peripheral Lesions Are Associated with Nonperfusion on Ultrawide Field Angiography.Ophthalmology. 2015 Dec;122(12):2465-72. doi: 10.1016/j.ophtha.2015.07.034. Epub 2015 Sep 6. Ophthalmology. 2015. PMID: 26350546
-
Diabetic retinopathy and ultrawide field imaging.Semin Ophthalmol. 2020 Jan 2;35(1):56-65. doi: 10.1080/08820538.2020.1729818. Epub 2020 Mar 13. Semin Ophthalmol. 2020. PMID: 32167854 Review.
-
Use of OCTA, FA, and Ultra-Widefield Imaging in Quantifying Retinal Ischemia: A Review.Asia Pac J Ophthalmol (Phila). 2018 Jan-Feb;7(1):46-51. doi: 10.22608/APO.201812. Epub 2018 Feb 13. Asia Pac J Ophthalmol (Phila). 2018. PMID: 29436208 Review.
Cited by
-
Ten-Year Trends of Fluorescein Angiography in Korea Using Data From the Health Insurance Review and Assessment Service.J Korean Med Sci. 2025 Apr 14;40(14):e39. doi: 10.3346/jkms.2025.40.e39. J Korean Med Sci. 2025. PMID: 40228558 Free PMC article.
-
Association between macrophage-like cell density and ischemia metrics in diabetic eyes.Exp Eye Res. 2023 Dec;237:109703. doi: 10.1016/j.exer.2023.109703. Epub 2023 Oct 28. Exp Eye Res. 2023. PMID: 38652673 Free PMC article.
-
Retinal non-perfusion: recognizing and defining what is important.Eye (Lond). 2024 Jun;38(9):1608-1609. doi: 10.1038/s41433-024-02981-x. Epub 2024 Mar 4. Eye (Lond). 2024. PMID: 38438795 Free PMC article. No abstract available.
-
Systemic Hypoxia Increases Retinal Blood Flow but Reduces the Oxygen Saturation Less in Peripheral Than in Macular Vessels in Normal Persons.Invest Ophthalmol Vis Sci. 2025 Jun 2;66(6):43. doi: 10.1167/iovs.66.6.43. Invest Ophthalmol Vis Sci. 2025. PMID: 40512486 Free PMC article.
-
Ultra-Widefield Retinal Optical Coherence Tomography (OCT) and Angio-OCT Using an Add-On Lens.Diagnostics (Basel). 2025 Jul 3;15(13):1697. doi: 10.3390/diagnostics15131697. Diagnostics (Basel). 2025. PMID: 40647696 Free PMC article.
References
-
- Early Treatment Diabetic Retinopathy Study Research Group . Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology. 1991;98(5)(suppl):786-806. doi:10.1016/S0161-6420(13)38012-9 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

