Clinical and Genetic Risk Factors for Acute Incident Venous Thromboembolism in Ambulatory Patients With COVID-19
- PMID: 35980616
- PMCID: PMC9389434
- DOI: 10.1001/jamainternmed.2022.3858
Clinical and Genetic Risk Factors for Acute Incident Venous Thromboembolism in Ambulatory Patients With COVID-19
Erratum in
-
Omission in Data Sharing Statement.JAMA Intern Med. 2022 Nov 1;182(11):1234. doi: 10.1001/jamainternmed.2022.5153. JAMA Intern Med. 2022. PMID: 36342480 Free PMC article. No abstract available.
Abstract
Importance: The risk of venous thromboembolism (VTE) in ambulatory COVID-19 is controversial. In addition, the association of vaccination with COVID-19-related VTE and relevant clinical and genetic risk factors remain to be elucidated.
Objective: To quantify the association between ambulatory COVID-19 and short-term risk of VTE, study the potential protective role of vaccination, and investigate clinical and genetic risk factors for post-COVID-19 VTE.
Design, setting, and participants: This population-based cohort study of patients with COVID-19 from UK Biobank included participants with SARS-CoV-2 infection that was confirmed by a positive polymerase chain test reaction result between March 1, 2020, and September 3, 2021, who were then propensity score matched to COVID-19-naive people during the same period. Participants with a history of VTE who used antithrombotic drugs (1 year before index dates) or tested positive in hospital were excluded.
Exposures: First infection with SARS-CoV-2, age, sex, ethnicity, socioeconomic status, obesity, vaccination status, and inherited thrombophilia.
Main outcomes and measures: The primary outcome was a composite VTE, including deep vein thrombosis or pulmonary embolism, which occurred 30 days after the infection. Hazard ratios (HRs) with 95% CIs were calculated using cause-specific Cox models.
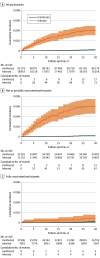
Results: In 18 818 outpatients with COVID-19 (10 580 women [56.2%]; mean [SD] age, 64.3 [8.0] years) and 93 179 matched uninfected participants (52 177 women [56.0%]; mean [SD] age, 64.3 [7.9] years), the infection was associated with an increased risk of VTE in 30 days (incidence rate of 50.99 and 2.37 per 1000 person-years for infected and uninfected people, respectively; HR, 21.42; 95% CI, 12.63-36.31). However, risk was substantially attenuated among the fully vaccinated (HR, 5.95; 95% CI, 1.82-19.5; interaction P = .02). In patients with COVID-19, older age, male sex, and obesity were independently associated with higher risk, with adjusted HRs of 1.87 (95% CI, 1.50-2.33) per 10 years, 1.69 (95% CI, 1.30-2.19), and 1.83 (95% CI, 1.28-2.61), respectively. Further, inherited thrombophilia was associated with an HR of 2.05 (95% CI, 1.15-3.66) for post-COVID-19 VTE.
Conclusions and relevance: In this population-based cohort study of patients with COVID-19, ambulatory COVID-19 was associated with a substantially increased risk of incident VTE, but this risk was greatly reduced in fully vaccinated people with breakthrough infection. Older age, male sex, and obesity were clinical risk factors for post-COVID-19 VTE; factor V Leiden thrombophilia was additionally associated with double the risk, comparable with the risk of 10-year aging. These findings may reinforce the need for vaccination, inform VTE risk stratification, and call for targeted VTE prophylaxis strategies for unvaccinated outpatients with COVID-19.
Conflict of interest statement
Figures

Comment in
-
Is There a Role for Thromboprophylaxis in Selected Outpatients With COVID-19?-Reply.JAMA Intern Med. 2023 Feb 1;183(2):169-170. doi: 10.1001/jamainternmed.2022.5881. JAMA Intern Med. 2023. PMID: 36534381 No abstract available.
-
Is There a Role for Thromboprophylaxis in Selected Outpatients With COVID-19?JAMA Intern Med. 2023 Feb 1;183(2):168-169. doi: 10.1001/jamainternmed.2022.5878. JAMA Intern Med. 2023. PMID: 36534390 No abstract available.
References
-
- Spyropoulos AC, Goldin M, Giannis D, et al. ; HEP-COVID Investigators . Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized Patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181(12):1612-1620. doi: 10.1001/jamainternmed.2021.6203 - DOI - PMC - PubMed
-
- Ortega-Paz L, Galli M, Capodanno D, et al. Safety and efficacy of different prophylactic anticoagulation dosing regimens in critically and non-critically ill patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother. Published online September 14, 2021. doi: 10.1093/ehjcvp/pvab070 - DOI - PMC - PubMed
-
- National Institutes of Health . Antithrombotic therapy in patients with COVID-19. Accessed December 11, 2021. https://www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-...
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

