Participation of Lower and Upper Middle-Income Countries in Clinical Trials Led by High-Income Countries
- PMID: 35980637
- PMCID: PMC9389348
- DOI: 10.1001/jamanetworkopen.2022.27252
Participation of Lower and Upper Middle-Income Countries in Clinical Trials Led by High-Income Countries
Erratum in
-
Omission in Title.JAMA Netw Open. 2022 Sep 1;5(9):e2235554. doi: 10.1001/jamanetworkopen.2022.35554. JAMA Netw Open. 2022. PMID: 36125816 Free PMC article. No abstract available.
Abstract
Importance: Many randomized clinical trials (RCTs) led by high-income countries (HICs) now enroll patients from lower middle-income countries (LMICs) and upper middle-income countries (UMICs). Although enrolling diverse populations promotes research collaborations, there are issues regarding which countries participate in RCTs and how this participation may contribute to global research.
Objective: To describe which UMICs and LMICs participate in RCTs led by HICs.
Design, setting, and participants: A cross-sectional study of all oncology RCTs published globally during January 1, 2014, to December 31, 2017, was conducted. The study cohort was restricted to RCTs led by HICs that enrolled participants from LMICs and UMICs. Study analyses were conducted in November 1, 2021, to May 31, 2022.
Main outcomes and measures: A bibliometric approach (Web of Science 2007-2017) was used to explore whether RCT participation was proportional to other measures of cancer research activity. Participation in RCTs (ie, percentage of RCTs in the cohort in which each LMIC and UMIC participated) was compared with country-level cancer research bibliometric output (ie, percentage of total cancer research bibliometric output from the same group of countries that came from a specific LMIC and UMIC).
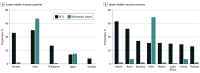
Results: Among the 636 HIC-led RCTs, 186 trials (29%) enrolled patients in LMICs (n = 84 trials involving 11 LMICs) and/or UMICs (n = 181 trials involving 26 UMICs). The most common participating LMICs were India (42 [50%]), Ukraine (39 [46%]), Philippines (23 [27%]), and Egypt (12 [14%]). The most common participating UMICs were Russia (115 [64%]), Brazil (94 [52%]), Romania (62 [34%]), China (56 [31%]), Mexico (56 [31%]), and South Africa (54 [30%]). Several LMICs are overrepresented in the cohort of RCTs based on proportional cancer research bibliometric output: Ukraine (46% of RCTs but 2% of cancer research bibliometric output), Philippines (27% RCTs, 1% output), and Georgia (8% RCTs, 0.2% output). Overrepresented UMICs include Russia (64% RCTs, 2% output), Romania (34% RCTs, 2% output), Mexico (31% RCTs, 2% output), and South Africa (30% RCTs, 1% output).
Conclusions and relevance: In this cross-sectional study, a substantial proportion of RCTs led by HICs enrolled patients in LMICs and UMICs. The LMICs and UMICs that participated in these trials did not match overall cancer bibliometric output as a surrogate for research ecosystem maturity. Reasons for this apparent discordance and how these data may inform future capacity-strengthening activities require further study.
Conflict of interest statement
Figures