Anti-seizure efficacy and retention rate of carbamazepine is highly variable in randomized controlled trials: A meta-analysis
- PMID: 35980668
- PMCID: PMC9712487
- DOI: 10.1002/epi4.12644
Anti-seizure efficacy and retention rate of carbamazepine is highly variable in randomized controlled trials: A meta-analysis
Abstract
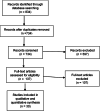
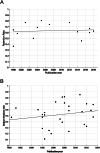
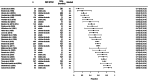
We sought to assess the anti-seizure efficacy of carbamazepine (CBZ) and retention rate (RR) in randomized, controlled trials (RCTs) in epilepsy. Our analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Inclusion criteria were monotherapy of CBZ in adequate dosage for epilepsy treatment and RCT duration of ≥3 months. Outcome measures were seizure freedom rate (SFR) and RR. Random-effects meta-analyses were performed to allow for comparison with other anti-seizure medications (ASMs). Thirty RCTs of 734 were included. SFR at last follow-up ranged from 11% at 36 months to 85% at 3 months. The aggregated SFR at 6 months was 58% (CI 49-66%) and 48% (CI 40-57%) at 12 months. The 6-month SFR among blinded studies was 55% (CI 43-66%), compared with 61% (CI 50-71%) in unblinded studies. The 12-month SFR was not significantly linked to the age of study participants. RR varied from 36% at 24 months to 81% at 6 months. When adjusting for blinding, the aggregated 6-month RR in blinded studies was 59% (CI 52-66%) vs 76% (CI 71-81%) in unblinded studies. The point estimates of SFR of all RCTs showed an upward time trend, with an increase of approximately 15% between the years 1981 and 2018. In conclusion, the SFR and RR of CBZ were highly variable in RCTs and especially affected by study duration and blinding. These results underscore the impact of the design of RCTs investigating ASM and may challenge the wide use of CBZ as a comparator.
Keywords: antiepileptic drug; antiseizure medication; clinical trial; focal epilepsy; monotherapy; study design.
© 2022 The Authors. Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
KOD has received speaker fees from Eisai, unrelated to this work. RS has received fees as speaker or for serving on the advisory board from Angelini, Arvelle, Bial, Desitin, Eisai, Janssen‐Cilag GmbH, LivaNova, Novartis, Precisis GmbH, UCB Pharma, UnEEG and Zogenix. These activities were not related to the content of this manuscript. C. Hoppe received software license fees and honoraria from Eisai Inc. unrelated to this work. The remaining authors have no conflicts of interest.
Figures


Similar articles
-
Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2019 Jul 18;7(7):CD001911. doi: 10.1002/14651858.CD001911.pub4. Cochrane Database Syst Rev. 2019. PMID: 31318037 Free PMC article.
-
Topiramate versus carbamazepine monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2019 Jun 24;6(6):CD012065. doi: 10.1002/14651858.CD012065.pub3. Cochrane Database Syst Rev. 2019. PMID: 31233229 Free PMC article.
-
Unblinded, randomized multicenter trial comparing lamotrigine and valproate combination with controlled-release carbamazepine monotherapy as initial drug regimen in untreated epilepsy.Seizure. 2018 Feb;55:17-24. doi: 10.1016/j.seizure.2017.12.008. Epub 2017 Dec 29. Seizure. 2018. PMID: 29324401 Clinical Trial.
-
Rufinamide add-on therapy for drug-resistant epilepsy.Cochrane Database Syst Rev. 2020 Nov 8;11(11):CD011772. doi: 10.1002/14651858.CD011772.pub3. Cochrane Database Syst Rev. 2020. PMID: 33179247 Free PMC article.
-
Anti-seizure medications for Lennox-Gastaut syndrome.Cochrane Database Syst Rev. 2021 Apr 7;4(4):CD003277. doi: 10.1002/14651858.CD003277.pub4. Cochrane Database Syst Rev. 2021. PMID: 33825230 Free PMC article.
Cited by
-
Establishing Clinically Relevant Specifications for Carbamazepine Tablets Using Physiologically Based Pharmacokinetic Modeling.AAPS J. 2025 May 2;27(4):87. doi: 10.1208/s12248-025-01074-1. AAPS J. 2025. PMID: 40316811
-
Efficacy and safety of add-on antiseizure medications for focal epilepsy: A network meta-analysis.Epilepsia Open. 2024 Aug;9(4):1550-1564. doi: 10.1002/epi4.12997. Epub 2024 Jun 18. Epilepsia Open. 2024. PMID: 38888005 Free PMC article.
-
Subchronic Treatment with CBZ Transiently Attenuates Its Anticonvulsant Activity in the Maximal Electroshock-Induced Seizure Test in Mice.Int J Mol Sci. 2024 Dec 18;25(24):13563. doi: 10.3390/ijms252413563. Int J Mol Sci. 2024. PMID: 39769325 Free PMC article.
References
-
- Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30‐year longitudinal cohort study [published correction appears in JAMA neurol. 2018 mar 1;75(3):384]. JAMA Neurol. 2018;75(3):279–86. - PMC - PubMed
-
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies [published correction appears in Epilepsia. 2010 Sep;51(9):1922]. Epilepsia. 2010;51(6):1069–77. 10.1111/j.1528-1167.2009.02397.x - DOI - PubMed
-
- Brodie MJ, Richens A, Yuen AW. Double‐blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. UK lamotrigine/carbamazepine monotherapy trial group [published correction appears in lancet 1995 mar 11;345(8950):662]. Lancet. 1995;345(8948):476–9. 10.1016/s0140-6736(95)90581-2 - DOI - PubMed
-
- Baulac M, Brodie MJ, Patten A, Segieth J, Giorgi L. Efficacy and tolerability of zonisamide versus controlled‐release carbamazepine for newly diagnosed partial epilepsy: a phase 3, randomised, double‐blind, non‐inferiority trial. Lancet Neurol. 2012;11(7):579–88. 10.1016/S1474-4422(12)70105-9 - DOI - PubMed

