B cell-intrinsic changes with age do not impact antibody-secreting cell formation but delay B cell participation in the germinal centre reaction
- PMID: 35980826
- PMCID: PMC9470890
- DOI: 10.1111/acel.13692
B cell-intrinsic changes with age do not impact antibody-secreting cell formation but delay B cell participation in the germinal centre reaction
Abstract
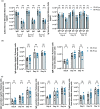
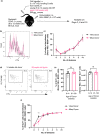
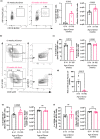
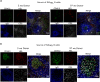
Vaccines typically protect against (re)infections by generating pathogen-neutralising antibodies. However, as we age, antibody-secreting cell formation and vaccine-induced antibody titres are reduced. Antibody-secreting plasma cells differentiate from B cells either early post-vaccination through the extrafollicular response or from the germinal centre (GC) reaction, which generates long-lived antibody-secreting cells. As the formation of both the extrafollicular antibody response and the GC requires the interaction of multiple cell types, the impaired antibody response in ageing could be caused by B cell intrinsic or extrinsic factors, or a combination of the two. Here, we show that B cells from older people do not have intrinsic defects in their proliferation and differentiation into antibody-secreting cells in vitro compared to those from the younger donors. However, adoptive transfer of B cells from aged mice to young recipient mice showed that differentiation into extrafollicular plasma cells was favoured at the expense of B cells entering the GC during the early stages of GC formation. In contrast, by the peak of the GC response, GC B cells derived from the donor cells of aged mice had expanded to the same extent as those from the younger donors. This indicates that age-related intrinsic B cell changes delay the GC response but are not responsible for the impaired antibody-secreting response or smaller peak GC response in ageing. Collectively, this study shows that B cells from aged individuals are not intrinsically defective in responding to stimulation and becoming antibody-secreting cells, implicating B cell-extrinsic factors as the primary cause of age-associated impairment in the humoral immunity.
Keywords: B cells; ageing; antibodies; vaccine response.
© 2022 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Targeting Vaccine-Induced Extrafollicular Pathway of B Cell Differentiation Improves Rabies Postexposure Prophylaxis.J Virol. 2017 Mar 29;91(8):e02435-16. doi: 10.1128/JVI.02435-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148792 Free PMC article.
-
Protective Humoral Immunity in the Central Nervous System Requires Peripheral CD19-Dependent Germinal Center Formation following Coronavirus Encephalomyelitis.J Virol. 2017 Nov 14;91(23):e01352-17. doi: 10.1128/JVI.01352-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931676 Free PMC article.
-
Early T Follicular Helper Cell Responses and Germinal Center Reactions Are Associated with Viremia Control in Immunized Rhesus Macaques.J Virol. 2019 Feb 5;93(4):e01687-18. doi: 10.1128/JVI.01687-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463978 Free PMC article.
-
Mechanisms underpinning poor antibody responses to vaccines in ageing.Immunol Lett. 2022 Jan;241:1-14. doi: 10.1016/j.imlet.2021.11.001. Epub 2021 Nov 10. Immunol Lett. 2022. PMID: 34767859 Free PMC article. Review.
-
Single-Cell Technologies for the Study of Antibody-Secreting Cells.Front Immunol. 2022 Jan 31;12:821729. doi: 10.3389/fimmu.2021.821729. eCollection 2021. Front Immunol. 2022. PMID: 35173713 Free PMC article. Review.
Cited by
-
Depletion of preexisting B-cell lymphoma 2-expressing senescent cells before vaccination impacts antigen-specific antitumor immune responses in old mice.Aging Cell. 2023 Dec;22(12):e14007. doi: 10.1111/acel.14007. Epub 2023 Nov 23. Aging Cell. 2023. PMID: 37997569 Free PMC article.
-
Enhancing thymic function improves T-cell reconstitution and immune responses in aged mice.PLoS Biol. 2025 Jul 28;23(7):e3003283. doi: 10.1371/journal.pbio.3003283. eCollection 2025 Jul. PLoS Biol. 2025. PMID: 40720380 Free PMC article.
-
The role of autoantibodies in bridging obesity, aging, and immunosenescence.Immun Ageing. 2024 Nov 30;21(1):85. doi: 10.1186/s12979-024-00489-2. Immun Ageing. 2024. PMID: 39616399 Free PMC article. Review.
-
B Cells from Aged Mice Do Not Have Intrinsic Defects in Affinity Maturation in Response to Immunization.J Immunol. 2023 Nov 15;211(10):1506-1515. doi: 10.4049/jimmunol.2300318. J Immunol. 2023. PMID: 37756528 Free PMC article.
-
TNF is a critical cytokine in age-related dry eye disease.Ocul Surf. 2023 Oct;30:119-128. doi: 10.1016/j.jtos.2023.08.004. Epub 2023 Aug 25. Ocul Surf. 2023. PMID: 37634571 Free PMC article.
References
-
- Attridge, K. , Kenefeck, R. , Wardzinski, L. , Qureshi, O. S. , Wang, C. J. , Manzotti, C. , Okkenhaug, K. , & Walker, L. S. (2014). IL‐21 promotes CD4 T cell responses by phosphatidylinositol 3‐kinase‐dependent upregulation of CD86 on B cells. The Journal of Immunology, 192, 2195–2201. - PMC - PubMed
-
- Breitbart, E. , Wang, X. , Leka, L. S. , Dallal, G. E. , Meydani, S. N. , & Stollar, B. D. (2002). Altered memory B‐cell homeostasis in human aging. Journals of Gerontology – Series A Biological Sciences and Medical Sciences, 57A, 304–311. - PubMed
-
- Brink, R. , Paus, D. , Bourne, K. , Hermes, J. R. , Gardam, S. , Phan, T. G. , & Chan, T. D. (2015). The SWHEL system for high‐resolution analysis of in vivo antigen‐specific T‐dependent B cell responses. Methods in Molecular Biology, 1291, 103–123. - PubMed
-
- Bums, E. A. , Lum, L. G. , L'hommedieu, G. , & Goodwin, J. S. (1993). Specific humoral immunity in the elderly: In vivo and in vitro response to vaccination. Journal of Gerontology: Biological Sciences, 48, 231–236. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

