Network modeling of dynamic brain interactions predicts emergence of neural information that supports human cognitive behavior
- PMID: 35980898
- PMCID: PMC9387855
- DOI: 10.1371/journal.pbio.3001686
Network modeling of dynamic brain interactions predicts emergence of neural information that supports human cognitive behavior
Abstract
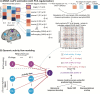
How cognitive task behavior is generated by brain network interactions is a central question in neuroscience. Answering this question calls for the development of novel analysis tools that can firstly capture neural signatures of task information with high spatial and temporal precision (the "where and when") and then allow for empirical testing of alternative network models of brain function that link information to behavior (the "how"). We outline a novel network modeling approach suited to this purpose that is applied to noninvasive functional neuroimaging data in humans. We first dynamically decoded the spatiotemporal signatures of task information in the human brain by combining MRI-individualized source electroencephalography (EEG) with multivariate pattern analysis (MVPA). A newly developed network modeling approach-dynamic activity flow modeling-then simulated the flow of task-evoked activity over more causally interpretable (relative to standard functional connectivity [FC] approaches) resting-state functional connections (dynamic, lagged, direct, and directional). We demonstrate the utility of this modeling approach by applying it to elucidate network processes underlying sensory-motor information flow in the brain, revealing accurate predictions of empirical response information dynamics underlying behavior. Extending the model toward simulating network lesions suggested a role for the cognitive control networks (CCNs) as primary drivers of response information flow, transitioning from early dorsal attention network-dominated sensory-to-response transformation to later collaborative CCN engagement during response selection. These results demonstrate the utility of the dynamic activity flow modeling approach in identifying the generative network processes underlying neurocognitive phenomena.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Comment in
-
Combined methods reveal task activation dynamics in human brain networks.PLoS Biol. 2022 Aug 19;20(8):e3001749. doi: 10.1371/journal.pbio.3001749. eCollection 2022 Aug. PLoS Biol. 2022. PMID: 35984785 Free PMC article.
Similar articles
-
The Functional Relevance of Task-State Functional Connectivity.J Neurosci. 2021 Mar 24;41(12):2684-2702. doi: 10.1523/JNEUROSCI.1713-20.2021. Epub 2021 Feb 4. J Neurosci. 2021. PMID: 33542083 Free PMC article.
-
Transcranial direct current stimulation changes resting state functional connectivity: A large-scale brain network modeling study.Neuroimage. 2016 Oct 15;140:174-87. doi: 10.1016/j.neuroimage.2016.02.015. Epub 2016 Feb 13. Neuroimage. 2016. PMID: 26883068
-
Distinct Patterns of Temporal and Directional Connectivity among Intrinsic Networks in the Human Brain.J Neurosci. 2017 Oct 4;37(40):9667-9674. doi: 10.1523/JNEUROSCI.1574-17.2017. Epub 2017 Sep 11. J Neurosci. 2017. PMID: 28893929 Free PMC article.
-
Mapping cognitive and emotional networks in neurosurgical patients using resting-state functional magnetic resonance imaging.Neurosurg Focus. 2020 Feb 1;48(2):E9. doi: 10.3171/2019.11.FOCUS19773. Neurosurg Focus. 2020. PMID: 32006946 Free PMC article. Review.
-
From connectome to cognition: The search for mechanism in human functional brain networks.Neuroimage. 2017 Oct 15;160:124-139. doi: 10.1016/j.neuroimage.2017.01.060. Epub 2017 Jan 26. Neuroimage. 2017. PMID: 28131891 Free PMC article. Review.
Cited by
-
Causally informed activity flow models provide mechanistic insight into network-generated cognitive activations.Neuroimage. 2023 Sep;278:120300. doi: 10.1016/j.neuroimage.2023.120300. Epub 2023 Jul 29. Neuroimage. 2023. PMID: 37524170 Free PMC article.
-
Regularized partial correlation provides reliable functional connectivity estimates while correcting for widespread confounding.bioRxiv [Preprint]. 2025 Aug 22:2023.09.16.558065. doi: 10.1101/2023.09.16.558065. bioRxiv. 2025. PMID: 40894659 Free PMC article. Preprint.
-
Artificial intelligence-powered intraoperative nerve monitoring: a visionary method to reduce facial nerve palsy in parotid surgery: an editorial.Ann Med Surg (Lond). 2023 Dec 15;86(2):635-637. doi: 10.1097/MS9.0000000000001612. eCollection 2024 Feb. Ann Med Surg (Lond). 2023. PMID: 38333244 Free PMC article. No abstract available.
-
Brain network dynamics reflect psychiatric illness status and transdiagnostic symptom profiles across health and disease.bioRxiv [Preprint]. 2025 May 28:2025.05.23.655864. doi: 10.1101/2025.05.23.655864. bioRxiv. 2025. PMID: 40502066 Free PMC article. Preprint.
-
Combined methods reveal task activation dynamics in human brain networks.PLoS Biol. 2022 Aug 19;20(8):e3001749. doi: 10.1371/journal.pbio.3001749. eCollection 2022 Aug. PLoS Biol. 2022. PMID: 35984785 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

