Comparison of 8- versus 12-weeks of glecaprevir/pibrentasvir for Taiwanese patients with hepatitis C and compensated cirrhosis in a real-world setting
- PMID: 35980912
- PMCID: PMC9387785
- DOI: 10.1371/journal.pone.0272567
Comparison of 8- versus 12-weeks of glecaprevir/pibrentasvir for Taiwanese patients with hepatitis C and compensated cirrhosis in a real-world setting
Abstract
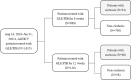
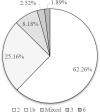
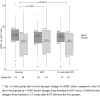
Real-world data on the effectiveness of glecaprevir/pibrentasvir (GLE/PIB) for patients with HCV infection and compensated cirrhosis is limited, especially for the 8-week regimen and in an Asian population. This retrospective study enrolled 159 consecutive patients with HCV and compensated cirrhosis who were treated with GLE/PIB at a single center in Taiwan. Sustained virological response (SVR) and adverse events (AEs) were evaluated. Among the 159 patients, 91 and 68 were treated with GLE/PIB for 8 and 12 weeks, respectively. In the per protocol analysis, both the 8- and 12-week groups achieved 100% SVR (87/87 vs. 64/64); and in the evaluable population analysis, 95.6% (87/91) of the 8-week group and 94.1% (64/68) of the 12-week group achieved SVR. The most commonly reported AEs, which included pruritus (15.4% vs. 26.5%), abdominal discomfort (9.9% vs. 5.9%), and skin rash (5.5% vs. 5.9%), were mild for the 8- and 12-week groups. Two patients in the 8-week group exhibited total bilirubin elevation over three times the upper normal limit. One of these two patients discontinued GLE/PIB treatment after 2 weeks but still achieved SVR. Both 8- and 12-week GLE/PIB treatments are safe and effective for patients of Taiwanese ethnicity with HCV and compensated cirrhosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Real-World Safety and Effectiveness of an 8-Week Regimen of Glecaprevir/Pibrentasvir in Patients with Hepatitis C and Cirrhosis.Adv Ther. 2024 Feb;41(2):744-758. doi: 10.1007/s12325-023-02748-y. Epub 2024 Jan 2. Adv Ther. 2024. PMID: 38169058 Free PMC article.
-
Real-world effectiveness of glecaprevir/pibrentasvir and ledipasvir/sofosbuvir for mixed genotype hepatitis C infection: A multicenter pooled analysis in Taiwan.J Viral Hepat. 2020 Sep;27(9):866-872. doi: 10.1111/jvh.13305. Epub 2020 May 12. J Viral Hepat. 2020. PMID: 32343472
-
Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis: The EXPEDITION-8 trial.J Hepatol. 2020 Mar;72(3):441-449. doi: 10.1016/j.jhep.2019.10.020. Epub 2019 Nov 2. J Hepatol. 2020. PMID: 31682879 Clinical Trial.
-
Real-World Clinical Practice Use of 8-Week Glecaprevir/Pibrentasvir in Treatment-Naïve Patients with Compensated Cirrhosis.Adv Ther. 2020 Sep;37(9):4033-4042. doi: 10.1007/s12325-020-01449-0. Epub 2020 Aug 4. Adv Ther. 2020. PMID: 32754824 Free PMC article. Review.
-
Glecaprevir/pibrentasvir expands reach while reducing cost and duration of hepatitis C virus therapy.Hepatol Int. 2018 May;12(3):214-222. doi: 10.1007/s12072-018-9873-y. Epub 2018 May 29. Hepatol Int. 2018. PMID: 29845496 Free PMC article. Review.
Cited by
-
Effectiveness and safety of 8-week glecaprevir/pibrentasvir in HCV treatment-naïve patients with compensated cirrhosis: real-world experience from Taiwan nationwide HCV registry.Hepatol Int. 2023 Jun;17(3):550-561. doi: 10.1007/s12072-023-10506-z. Epub 2023 Mar 27. Hepatol Int. 2023. PMID: 36973633 Free PMC article.
-
Real-World Efficacy and Safety of Universal 8-Week Glecaprevir/Pibrentasvir for Treatment-Naïve Patients from a Nationwide HCV Registry in Taiwan.Infect Dis Ther. 2024 Jun;13(6):1199-1213. doi: 10.1007/s40121-024-00968-5. Epub 2024 Apr 28. Infect Dis Ther. 2024. PMID: 38679663 Free PMC article.
-
Pruritus in Chronic Cholestatic Liver Diseases, Especially in Primary Biliary Cholangitis: A Narrative Review.Int J Mol Sci. 2025 Feb 22;26(5):1883. doi: 10.3390/ijms26051883. Int J Mol Sci. 2025. PMID: 40076514 Free PMC article. Review.
References
-
- Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: A community-based long-term prospective study. J Inf Dis 2010;206:469–477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical