Multi-center matched cohort study of convalescent plasma for hospitalized patients with COVID-19
- PMID: 35980913
- PMCID: PMC9387784
- DOI: 10.1371/journal.pone.0273223
Multi-center matched cohort study of convalescent plasma for hospitalized patients with COVID-19
Abstract
Background: Although frequently used in the early pandemic, data on the effectiveness of COVID-19 convalescent plasma (CCP) remain mixed. We investigated the effectiveness and safety of CCP in hospitalized COVID-19 patients in real-world practices during the first two waves of the pandemic in a multi-hospital healthcare system in Texas.
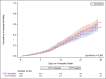
Methods and findings: Among 11,322 hospitalized patients with confirmed COVID-19 infection from July 1, 2020 to April 15, 2021, we included patients who received CCP and matched them with those who did not receive CCP within ±2 days of the transfusion date across sites within strata of sex, age groups, days and use of dexamethasone from hospital admission to the match date, and oxygen requirements 4-12 hours prior to the match date. Cox proportional hazards model estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for effectiveness outcomes in a propensity score 1:1 matched cohort. Pre-defined safety outcomes were described. We included 1,245 patients each in the CCP treated and untreated groups. Oxygen support was required by 93% of patients at the baseline. The pre-defined primary effectiveness outcome of 28-day in-hospital all-cause mortality (HR = 0.85; 95%CI: 0.66,1.10) were similar between treatment groups. Sensitivity and stratified analyses found similar null results. CCP-treated patients were less likely to be discharged alive (HR = 0.82; 95%CI: 0.74, 0.91), and more likely to receive mechanical ventilation (HR = 1.48; 95%CI: 1.12, 1.96). Safety outcomes were rare and similar between treatment groups.
Conclusion: The findings in this large, matched cohort of patients hospitalized with COVID-19 and mostly requiring oxygen support at the time of treatment, do not support a clinical benefit in 28-day in-hospital all-cause mortality for CCP. Future studies should assess the potential benefits with specifically high-titer units in perhaps certain subgroups of patients (e.g. those with early disease or immunocompromised).
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures



References
-
- US Food and Drug Administration. Clinical Memorandum Re: EUA 26382 2021. [cited March 25 2022]. Available from: https://www.fda.gov/media/155159/download.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

