MicroRNA-155 acts as an anti-inflammatory factor in orbital fibroblasts from Graves' orbitopathy by repressing interleukin-2-inducible T-cell kinase
- PMID: 35980936
- PMCID: PMC9387810
- DOI: 10.1371/journal.pone.0270416
MicroRNA-155 acts as an anti-inflammatory factor in orbital fibroblasts from Graves' orbitopathy by repressing interleukin-2-inducible T-cell kinase
Abstract
To investigate the role of microRNA (miR)-155 in inflammation in an in-vitro model of Graves' orbitopathy (GO). The expression levels of miR-155 were compared between GO and non-GO orbital tissues. The effects of inflammatory stimulation of interleukin (IL)-1β and tumour necrosis factor alpha (TNF-α) on miR-155 expression on GO and non-GO orbital fibroblasts (OFs) were investigated. The effects of miR-155 mimics and inhibitors of inflammatory proteins and IL-2-inducible T-cell kinase (ITK) expression were examined, along with those related to the knockdown of ITK with siITK transfection on inflammatory proteins. We also examined how ITK inhibitors affect miR-155 expression in GO and non-GO OFs. The expression levels of miR-155 were higher in GO orbital tissues than in non-GO tissue. The overexpression of miR-155 was induced by IL-1β and TNF-α in OFs from GO and non-GO patients. IL-1β-induced IL-6 (ICAM1) protein production was significantly reduced (increased) by miR-155 mimics and inhibitors. The mRNA and protein levels of ITK were downregulated by overexpressed miR-155 via miR-155 mimics. Knockdown of ITK via siITK transfection induced a decrease in the expression levels of ITK, IL-17, IL-6, IL-1β, and TNF-α protein. The expression of miR-155 was significantly downregulated by treatment with ITK inhibitors and Bruton's tyrosine kinase (BTK)/ITK dual inhibitors in a time-dependent manner. Our results indicated a potential relationship between miR-155 and ITK in the context of GO OFs. The overexpression of miR-155 repressed ITK expression and relieved inflammation. Thus, miR-155 appears to have anti-inflammatory effects in GO OFs. This discovery provides a new concept for developing GO treatment therapeutics.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





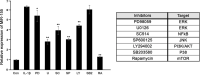

Similar articles
-
Therapeutic effect of selective interleukin-2-inducible tyrosine kinase inhibitor in orbital fibroblasts from patients with Graves' orbitopathy.Endocr J. 2024 Sep 2;71(9):851-861. doi: 10.1507/endocrj.EJ23-0729. Epub 2024 Jun 13. Endocr J. 2024. PMID: 38866492
-
Impact of ibrutinib on inflammation in a mouse model of Graves' orbitopathy.Front Endocrinol (Lausanne). 2024 Aug 30;15:1420024. doi: 10.3389/fendo.2024.1420024. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39280007 Free PMC article.
-
Role of miR-146a in the Regulation of Inflammation in an In Vitro Model of Graves' Orbitopathy.Invest Ophthalmol Vis Sci. 2016 Aug 1;57(10):4027-34. doi: 10.1167/iovs.16-19213. Invest Ophthalmol Vis Sci. 2016. PMID: 27494344
-
Mechanisms That Underly T Cell Immunity in Graves' Orbitopathy.Front Endocrinol (Lausanne). 2021 Apr 1;12:648732. doi: 10.3389/fendo.2021.648732. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33868176 Free PMC article. Review.
-
Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves' ophthalmopathy.Exp Eye Res. 2016 Jan;142:83-91. doi: 10.1016/j.exer.2015.02.007. Exp Eye Res. 2016. PMID: 26675405 Review.
Cited by
-
Comparative study of the effects of baicalin and probenecid on microRNA expression profiles in porcine aortic vascular endothelial cells infected by Glaesserella parasuis.BMC Vet Res. 2025 Apr 2;21(1):237. doi: 10.1186/s12917-025-04702-2. BMC Vet Res. 2025. PMID: 40176019 Free PMC article.
-
The miR-320a/PRDX3 Axis Alleviates the Oxidative Stress and Fibrotic Alterations in Fibroblasts in Thyroid Eye Disease.Invest Ophthalmol Vis Sci. 2025 Jul 1;66(9):41. doi: 10.1167/iovs.66.9.41. Invest Ophthalmol Vis Sci. 2025. PMID: 40657967 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

