Acyloxyacyl hydrolase regulates microglia-mediated pelvic pain
- PMID: 35980963
- PMCID: PMC9387837
- DOI: 10.1371/journal.pone.0269140
Acyloxyacyl hydrolase regulates microglia-mediated pelvic pain
Abstract
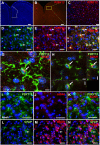
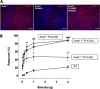
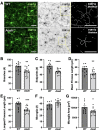
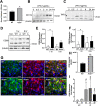
Chronic pelvic pain conditions such as interstitial cystitis/bladder pain syndrome (IC/BPS) remain clinical and mechanistic enigmas. Microglia are resident immune cells of the central nervous system (CNS) that respond to changes in the gut microbiome, and studies have linked microglial activation to acute and chronic pain in a variety of models, including pelvic pain. We have previously reported that mice deficient for the lipase acyloxyacyl hydrolase (AOAH) develop pelvic allodynia and exhibit symptoms, comorbidities, and gut dysbiosis mimicking IC/BPS. Here, we assessed the role of AOAH in microglial activation and pelvic pain. RNAseq analyses using the ARCHS4 database and confocal microscopy revealed that AOAH is highly expressed in wild type microglia but at low levels in astrocytes, suggesting a functional role for AOAH in microglia. Pharmacologic ablation of CNS microglia with PLX5622 resulted in decreased pelvic allodynia in AOAH-deficient mice and resurgence of pelvic pain upon drug washout. Skeletal analyses revealed that AOAH-deficient mice have an activated microglia morphology in the medial prefrontal cortex and paraventricular nucleus, brain regions associated with pain modulation. Because microglia express Toll-like receptors and respond to microbial components, we also examine the potential role of dysbiosis in microglial activation. Consistent with our hypothesis of microglia activation by leakage of gut microbes, we observed increased serum endotoxins in AOAH-deficient mice and increased activation of cultured BV2 microglial cells by stool of AOAH-deficient mice. Together, these findings demonstrate a role for AOAH in microglial modulation of pelvic pain and thus identify a novel therapeutic target for IC/BPS.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

