Closed Aromatic Tubes-Capsularenes
- PMID: 35981224
- PMCID: PMC9825917
- DOI: 10.1002/anie.202211304
Closed Aromatic Tubes-Capsularenes
Abstract
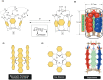
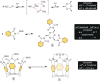
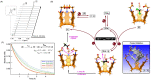
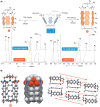
In this study, we describe a synthetic method for incorporating arenes into closed tubes that we name capsularenes. First, we prepared vase-shaped molecular baskets 4-7. The baskets comprise a benzene base fused to three bicycle[2.2.1]heptane rings that extend into phthalimide (4), naphthalimide (6), and anthraceneimide sides (7), each carrying a dimethoxyethane acetal group. In the presence of catalytic trifluoroacetic acid (TFA), the acetals at top of 4, 6 and 7 change into aliphatic aldehydes followed by their intramolecular cyclization into 1,3,5-trioxane (1 H NMR spectroscopy). Such ring closure is nearly a quantitative process that furnishes differently sized capsularenes 1 (0.7×0.9 nm), 8 (0.7×1.1 nm;) and 9 (0.7×1.4 nm;) characterized by X-Ray crystallography, microcrystal electron diffraction, UV/Vis, fluorescence, cyclic voltammetry, and thermogravimetry. With exceptional rigidity, unique topology, great thermal stability, and perhaps tuneable optoelectronic characteristics, capsularenes hold promise for the construction of novel organic electronic devices.
Keywords: Acenes; Aromatic Compounds; Molecular Electronics; Supramolecular Catalysis; Trioxanes.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

