CXCL1-CXCR2 signalling mediates hypertensive retinopathy by inducing macrophage infiltration
- PMID: 35981418
- PMCID: PMC9418605
- DOI: 10.1016/j.redox.2022.102438
CXCL1-CXCR2 signalling mediates hypertensive retinopathy by inducing macrophage infiltration
Abstract
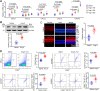
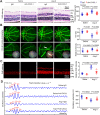
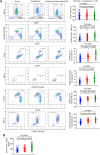
Inflammation plays an important role in hypertensive retinal vascular injury and subsequent retinopathy. Monocyte chemotaxis via CXCL1-CXCR2 binding has been implicated in various cardiovascular diseases, but the function of CXCL1-CXCR2 signalling involved in retinopathy, which was investigated as angiotensin II (Ang II)-induced retinopathy, is unclear. In our study, we established a hypertensive retinopathy (HR) model by Ang II infusion (3000 ng/min/kg) for 3 weeks. To determine the involvement of CXCR2 signalling, we used CXCR2 knockout (KO) mice or C57BL/6J wild-type (WT) mice as experimental subjects. The mice were treated with a CXCL1 neutralizing antibody or SB225002 (the specific CXCR2 inhibitor). Our results showed that after Ang II treatment, the mRNA levels of CXCL1 and CXCR2 and the number of CXCR2+ inflammatory cells were significantly elevated. Conversely, unlike in the IgG control group, the CXCL1 neutralizing antibody greatly reduced the increase in central retinal thickness induced by Ang II infusion, arteriolar remodelling, superoxide production, and retinal dysfunction in WT mice. Furthermore, Ang II infusion induced arteriolar remodelling, infiltration of Iba1+ macrophages, the production of oxidative stress, and retinal dysfunction, but the symptoms were ameliorated in CXCR2 KO mice and SB225002-treated mice. These protective effects were related to the reduction in the number of CXCR2+ immune cells, particularly macrophages, and the decrease in proinflammatory cytokine (IL-1β, IL-6, TNF-ɑ, and MCP-1) expression in Ang II-treated retinas. Notably, serum CXCL1 levels and the number of CXCR2+ monocytes/neutrophils were higher in HR patients than in healthy controls. In conclusion, this study provides new evidence that the CXCL1-CXCR2 axis plays a vital role in the pathogenesis of hypertensive retinopathy, and selective blockade of CXCL1-CXCR2 activation may be a potential treatment for HR.
Keywords: CXCL1; CXCR2; Hypertensive retinopathy; Inflammation; Macrophages.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no competing interests.
Figures





References
-
- Wenzel P., Knorr M., Kossmann S., Stratmann J., Hausding M., Schuhmacher S., et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124(12):1370–1381. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

