Effects of Surface Prereacted Glass on Saliva-Derived Polymicrobial Biofilms in an Active Attachment Biofilm Model
- PMID: 35981515
- PMCID: PMC9677873
- DOI: 10.1159/000526530
Effects of Surface Prereacted Glass on Saliva-Derived Polymicrobial Biofilms in an Active Attachment Biofilm Model
Abstract
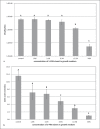
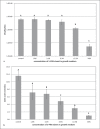
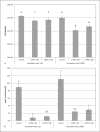
Bioactive restorative materials are being developed to either influence the de/remineralization balance of the dental hard tissues locally or to release components that interact with the oral microbiota. Surface prereacted glass (S-PRG, Shofu, Japan) is a material that may influence both processes. S-PRG releases fluoride, which can interact with the de/remineralization process, and a range of other compounds that may influence the oral microbiota. In the current study, several experiments were performed to investigate the potential of S-PRG to influence both the growth and lactic acid production of saliva-derived polymicrobial biofilms. Biofilm formation was studied using the Amsterdam Active Attachment model. An eluate of the S-PRG particles was tested by adding it to the growth medium or by exposing the biofilms to it for 1 h. The effect of S-PRG particles was tested by adding the particles to the growth medium. The current experiments showed that the presence of S-PRG eluate in the medium influenced biofilm growth and lactic acid production even at low concentrations. The composition of the biofilms changed in the presence of S-PRG eluate, even at concentrations of S-PRG eluate at which biofilm viability was not affected. Treatment of developing biofilms with S-PRG eluate did neither show an effect on biofilm viability nor on lactic acid production. The addition of S-PRG particles to the growth medium resulted in both a lower biofilm viability and lower lactic acid production, indicating that the release of ions from the particles was fast enough to influence biofilm formation. From the current experiments, it can be concluded that S-PRG has the potential to influence biofilm growth, but the presence of the released ions during biofilm formation is required to show an effect.
Keywords: Antimicrobial agents; Biofilms; Microbiology; Next-generation sequencing; Surface prereacted glass.
© 2022 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Rob A.M. Exterkate and Wim Crielaard received funding from Shofu Inc. to perform the studies in this manuscript. Both authors have no personal relationship with Shofu Inc.
Figures


References
-
- Amaechi BT, Key MC, Balu S, Okoye LO, Gakunga PT. Evaluation of the caries-preventive effect of toothpaste containing surface prereacted glass-ionomer filler. J Investig Clin Dent. 2017;8((4)):e12249. - PubMed
-
- Exterkate RAM, Crielaard W, Ten Cate JM. Different response to amine fluoride by Streptococcus mutans and polymicrobial biofilms in a novel high-throughput active attachment model. Caries Res. 2010;44((4)):372–379. - PubMed
-
- Fujimoto Y, Iwasa M, Murayama R, Miyazaki M, Nagafuji A, Nakatsuka T. Detection of ions released from S-PRG fillers and their modulation effect. Dental Mater J. 2010;29((4)):392–397. - PubMed
-
- Hahnel S, Wastl DS, Schneider-Feyrer S, Giessibl FJ, Brambilla E, Cazzaniga G, et al. Streptococcus mutans biofilm formation and release of fluoride from experimental resin-based composites depending on surface treatment and S-PRG filler particle fraction. J Adhes Dent. 2014;16((4)):313–321. - PubMed
-
- Hotta M, Morikawa T, Tamura D, Kusakabe S. Adherence of Streptococcus sanguinis and Streptococcus mutans to saliva-coated S-PRG resin blocks. Dent Mater J. 2014;33((2)):261–267. - PubMed

