Substandard and falsified antibiotics: neglected drivers of antimicrobial resistance?
- PMID: 35981806
- PMCID: PMC9394205
- DOI: 10.1136/bmjgh-2022-008587
Substandard and falsified antibiotics: neglected drivers of antimicrobial resistance?
Abstract
Objectives: Antimicrobial resistance (AMR) is a significant global health threat with substandard and falsified (SF) antibiotics being neglected contributing factors. With their relationships poorly understood, more research is needed in order to determine how interventions to reduce SF antibiotics should be ranked as priorities in national AMR action plans. We assessed the evidence available on the global prevalence of SF antibiotics, examined the quality of the evidence and discussed public health impact.
Materials/methods: We searched PubMed, Embase, Google and Google Scholar for publications on antibiotic quality up to 31 December 2020. Publications reporting on the prevalence of SF antibiotics were evaluated for quantitative analysis and assessed using the Medicines Quality Assessment Reporting Guidelines.
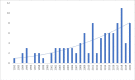
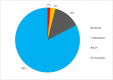
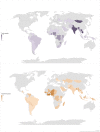
Results: Of the 10 137 screened publications, 648 were relevant to antibiotic quality. One hundred and six (16.4%) surveys, published between 1992 and 2020 and conducted mainly in low-income and middle-income countries (LMICs) (89.9% (480/534) of the data points), qualified for quantitative analysis. The total number of samples tested for quality in prevalence surveys was 13 555, with a median (Q1-Q3) number of samples per survey of 47 (21-135). Of the 13 555 samples, 2357 (17.4%) failed at least one quality test and the median failure frequency (FF) per survey was 19.6% (7.6%-35.0%). Amoxicillin, sulfamethoxazole-trimethoprim and ciprofloxacin were the most surveyed antibiotics, with FF of 16.1% (355/2208), 26.2% (329/1255) and 10.4% (366/3511), respectively. We identified no SF survey data for antibiotics in the WHO 'Reserve' group. The mean Medicine Quality Assessment Reporting Guidelines score was 11 (95% CI 10.1 to 12.2) out of 26.
Conclusions: SF antibiotics are widely spread with higher prevalence in LMICs. The quality of the evidence is poor, and these data are not generalisable that 17.4% of global antibiotic supply is SF. However, the evidence we have suggests that interventions to enhance regulatory, purchasing and financial mechanisms to improve the global antibiotic supply are needed.
Prospero registration number: CRD42019124988.
Keywords: medical microbiology; pharmacology; public Health.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Antibiotics Market Size, Share & Trends Analysis Report By Action Mechanism (Protein, DNA, RNA, Cell Wall Synthesis Inhibitors), By Drug Class (Penicillin, Cephalosporins, Fluoroquinolones), And Segment Forecasts, 2019 - 2026. 2019:1–119. Available: https://www.grandviewresearch.com/industry-analysis/antibiotic-market [Accessed 29 July 2022].
-
- World Health Organization . The top 10 causes of death, 2020. Available: http://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-... [Accessed 28 May 2021].
-
- World Health Organization . Member State mechanism on substandard / spurious / falsely - labelled / falsified / counterfeit medical products Report by the Director - General. WHO Technical Report Series 2017;20:1–42.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
