Continuous KRT: A Contemporary Review
- PMID: 35981873
- PMCID: PMC10103212
- DOI: 10.2215/CJN.04350422
Continuous KRT: A Contemporary Review
Abstract
AKI is a common complication of critical illness and is associated with substantial morbidity and risk of death. Continuous KRT comprises a spectrum of dialysis modalities preferably used to provide kidney support to patients with AKI who are hemodynamically unstable and critically ill. The various continuous KRT modalities are distinguished by different mechanisms of solute transport and use of dialysate and/or replacement solutions. Considerable variation exists in the application of continuous KRT due to a lack of standardization in how the treatments are prescribed, delivered, and optimized to improve patient outcomes. In this manuscript, we present an overview of the therapy, recent clinical trials, and outcome studies. We review the indications for continuous KRT and the technical aspects of the treatment, including continuous KRT modality, vascular access, dosing of continuous KRT, anticoagulation, volume management, nutrition, and continuous KRT complications. Finally, we highlight the need for close collaboration of a multidisciplinary team and development of quality assurance programs for the provision of high-quality and effective continuous KRT.
Copyright © 2022 by the American Society of Nephrology.
Conflict of interest statement
J.A. Neyra reports serving on the editorial boards of
Figures


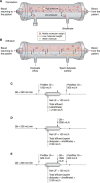
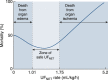
References
-
- Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA: Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015 - PubMed
-
- Wald R, McArthur E, Adhikari NK, Bagshaw SM, Burns KE, Garg AX, Harel Z, Kitchlu A, Mazer CD, Nash DM, Scales DC, Silver SA, Ray JG, Friedrich JO: Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: A population-based cohort study. Am J Kidney Dis 65: 870–877, 2015 - PubMed
-
- Uchino S Kellum JA Bellomo R Doig GS Morimatsu H Morgera S Schetz M Tan I Bouman C Macedo E Gibney N Tolwani A Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 - PubMed
-
- Palevsky PM Zhang JH O’Connor TZ Chertow GM Crowley ST Choudhury D Finkel K Kellum JA Paganini E Schein RM Smith MW Swanson KM Thompson BT Vijayan A Watnick S Star RA Peduzzi P; VA/NIH Acute Renal Failure Trial Network : Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

