Antigen-Dependent Inducible T-Cell Reporter System for PET Imaging of Breast Cancer and Glioblastoma
- PMID: 35981900
- PMCID: PMC9841254
- DOI: 10.2967/jnumed.122.264284
Antigen-Dependent Inducible T-Cell Reporter System for PET Imaging of Breast Cancer and Glioblastoma
Abstract
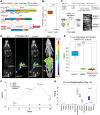
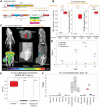
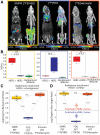
For the past several decades, chimeric antigen receptor T-cell therapies have shown promise in the treatment of cancers. These treatments would greatly benefit from companion imaging biomarkers to follow the trafficking of T cells in vivo. Methods: Using synthetic biology, we engineered T cells with a chimeric receptor synthetic intramembrane proteolysis receptor (SNIPR) that induces overexpression of an exogenous reporter gene cassette on recognition of specific tumor markers. We then applied a SNIPR-based PET reporter system to 2 cancer-relevant antigens, human epidermal growth factor receptor 2 (HER2) and epidermal growth factor receptor variant III (EGFRvIII), commonly expressed in breast and glial tumors, respectively. Results: Antigen-specific reporter induction of the SNIPR PET T cells was confirmed in vitro using green fluorescent protein fluorescence, luciferase luminescence, and the HSV-TK PET reporter with 9-(4-18F-fluoro-3-[hydroxymethyl]butyl)guanine ([18F]FHBG). T cells associated with their target antigens were successfully imaged using PET in dual-xenograft HER2+/HER2- and EGFRvIII+/EGFRvIII- animal models, with more than 10-fold higher [18F]FHBG signals seen in antigen-expressing tumors versus the corresponding controls. Conclusion: The main innovation found in this work was PET detection of T cells via specific antigen-induced signals, in contrast to reporter systems relying on constitutive gene expression.
Keywords: CAR T; PET; SNIPR; cancer antigens; reporter.
© 2023 by the Society of Nuclear Medicine and Molecular Imaging.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
