The role of the CBM complex in allergic inflammation and disease
- PMID: 35981904
- PMCID: PMC9643607
- DOI: 10.1016/j.jaci.2022.06.023
The role of the CBM complex in allergic inflammation and disease
Abstract
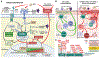
The caspase activation and recruitment domain-coiled-coil (CARD-CC) family of proteins-CARD9, CARD10, CARD11, and CARD14-is collectively expressed across nearly all tissues of the body and is a crucial mediator of immunologic signaling as part of the CARD-B-cell lymphoma/leukemia 10-mucosa-associated lymphoid tissue lymphoma translocation protein 1 (CBM) complex. Dysfunction or dysregulation of CBM proteins has been linked to numerous clinical manifestations known as "CBM-opathies." The CBM-opathy spectrum encompasses diseases ranging from mucocutaneous fungal infections and psoriasis to combined immunodeficiency and lymphoproliferative diseases; however, there is accumulating evidence that the CARD-CC family members also contribute to the pathogenesis and progression of allergic inflammation and allergic diseases. Here, we review the 4 CARD-CC paralogs, as well as B-cell lymphoma/leukemia 10 and mucosa-associated lymphoid tissue lymphoma translocation protein 1, and their individual and collective roles in the pathogenesis and progression of allergic inflammation and 4 major allergic diseases (allergic asthma, atopic dermatitis, food allergy, and allergic rhinitis).
Keywords: Allergy; BCL10; CARD; CARD10; CARD11; CARD14; CARD9; CBM-opathy; MALT1; allergic rhinitis; asthma; atopic dermatitis; food allergy.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures






References
-
- Burks AW, Holgate ST, O’Hehir RE, Broide DH, Bacharier LB, Hershey GKK, et al. Middleton’s allergy: principles and practice [Internet]. 2020. [cited 2020 Mar 25]. Available from: https://www.clinicalkey.com/dura/browse/bookChapter/3-s2.0-C20161002419
-
- Akdis CA, Arkwright PD, Brüggen MC, Busse W, Gadina M, Guttman-Yassky E, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582–605. - PubMed
-
- Davidson WF, Leung DYM, Beck LA, Berin CM, Boguniewicz M, Busse WW, et al. Report from the National Institute of Allergy and Infectious Diseases workshop on “Atopic dermatitis and the atopic march: Mechanisms and interventions.” Journal of Allergy and Clinical Immunology. 2019. Mar 1;143(3):894–913. - PMC - PubMed
-
- Regulation Mellett M. and dysregulation of CARD14 signalling and its physiological consequences in inflammatory skin disease. Cellular Immunology. 2020. Jun 13;104147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

