Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota
- PMID: 35982037
- PMCID: PMC9388534
- DOI: 10.1038/s41467-022-32015-7
Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota
Abstract
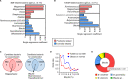
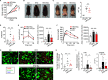
The gut microbiome is an important determinant in various diseases. Here we perform a cross-sectional study of Japanese adults and identify the Blautia genus, especially B. wexlerae, as a commensal bacterium that is inversely correlated with obesity and type 2 diabetes mellitus. Oral administration of B. wexlerae to mice induce metabolic changes and anti-inflammatory effects that decrease both high-fat diet-induced obesity and diabetes. The beneficial effects of B. wexlerae are correlated with unique amino-acid metabolism to produce S-adenosylmethionine, acetylcholine, and L-ornithine and carbohydrate metabolism resulting in the accumulation of amylopectin and production of succinate, lactate, and acetate, with simultaneous modification of the gut bacterial composition. These findings reveal unique regulatory pathways of host and microbial metabolism that may provide novel strategies in preventive and therapeutic approaches for metabolic disorders.
© 2022. The Author(s).
Conflict of interest statement
The authors of this manuscript have the following potential conflicts of interest: M.S., Y.O., H.S., and Y.Y. are employees of Noster, Inc. (Kyoto, Japan). Other authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

