Utilising low-cost, easy-to-use microscopy techniques for early peritonitis infection screening in peritoneal dialysis patients
- PMID: 35982214
- PMCID: PMC9388639
- DOI: 10.1038/s41598-022-18380-9
Utilising low-cost, easy-to-use microscopy techniques for early peritonitis infection screening in peritoneal dialysis patients
Abstract
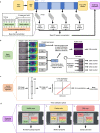
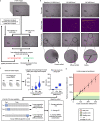
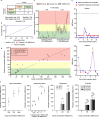
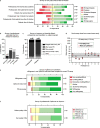
Peritoneal dialysis (PD) patients are at high risk for peritonitis, an infection of the peritoneum that affects 13% of PD users annually. Relying on subjective peritonitis symptoms results in delayed treatment, leading to high hospitalisation costs, peritoneal scarring, and premature transition to haemodialysis. We have developed and tested a low-cost, easy-to-use technology that uses microscopy and image analysis to screen for peritonitis across the effluent drain tube. Compared to other technologies, our prototype is made from off-the-shelf, low-cost materials. It can be set up quickly and key stakeholders believe it can improve the overall PD experience. We demonstrate that our prototype classifies infection-indicating and healthy white blood cell levels in clinically collected patient effluent with 94% accuracy. Integration of our technology into PD setups as a screening tool for peritonitis would enable earlier physician notification, allowing for prompt diagnosis and treatment to prevent hospitalisations, reduce scarring, and increase PD longevity. Our findings demonstrate the versatility of microscopy and image analysis for infection screening and are a proof of principle for their future applications in health care.
© 2022. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: M.B., J.K., A.B., and E.G. are inventors on a Patent Cooperation Treaty (PCT) international patent covering the microscopy-based white blood cell detection method and the device’s mechanical and computational design (International Publication No. WO 2022/165270), which was published on August 4, 2022. The following authors declare no competing interests: R.V., A.J., S.S., C.W., and K.S.
Figures

Similar articles
-
Peritoneal Cell-free DNA: an innovative method for determining acute cell damage in peritoneal membrane and for monitoring the recovery process after peritonitis.J Nephrol. 2016 Feb;29(1):111-8. doi: 10.1007/s40620-015-0212-2. Epub 2015 May 27. J Nephrol. 2016. PMID: 26012380
-
Surgical peritonitis in patients on peritoneal dialysis.Perit Dial Int. 2008 Jul-Aug;28(4):331-4. Perit Dial Int. 2008. PMID: 18556371 Review.
-
The Role of NGAL in Peritoneal Dialysis Effluent in Early Diagnosis of Peritonitis: Case-Control Study in Peritoneal Dialysis Patients.Perit Dial Int. 2015 Sep-Oct;35(5):559-65. doi: 10.3747/pdi.2013.00300. Epub 2014 Nov 13. Perit Dial Int. 2015. PMID: 25395499 Free PMC article.
-
Factors increasing severity of peritonitis in long-term peritoneal dialysis patients.Adv Ren Replace Ther. 1998 Jul;5(3):185-93. doi: 10.1016/s1073-4449(98)70031-7. Adv Ren Replace Ther. 1998. PMID: 9686629 Review.
-
Differences in hospitalisation between peritoneal dialysis and haemodialysis patients.Eur J Clin Invest. 2022 Jun;52(6):e13758. doi: 10.1111/eci.13758. Epub 2022 Feb 15. Eur J Clin Invest. 2022. PMID: 35129213 Free PMC article.
Cited by
-
Diagnostic Accuracy of MMP-8 and IL-6-Based Point-of-Care Testing to Detect Peritoneal Dialysis-Related Peritonitis: A Single-Center Experience.Diagnostics (Basel). 2024 May 27;14(11):1113. doi: 10.3390/diagnostics14111113. Diagnostics (Basel). 2024. PMID: 38893639 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

