Ecological analysis of Pavlovian fear conditioning in rats
- PMID: 35982246
- PMCID: PMC9388582
- DOI: 10.1038/s42003-022-03802-1
Ecological analysis of Pavlovian fear conditioning in rats
Abstract
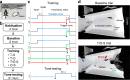
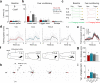
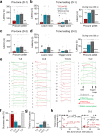
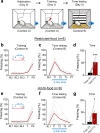
Pavlovian fear conditioning, which offers the advantage of simplicity in both the control of conditional and unconditional stimuli (CS, US) presentation and the analysis of specific conditional and unconditional responses (CR, UR) in a controlled laboratory setting, has been the standard model in basic and translational fear research. Despite 100 years of experiments, the utility of fear conditioning has not been trans-situationally validated in real-life contexts. We thus investigated whether fear conditioning readily occurs and guides the animal's future behavior in an ecologically-relevant environment. To do so, Long-Evans rats foraging for food in an open arena were presented with a tone CS paired with electric shock US to their dorsal neck/body that instinctively elicited escape UR to the safe nest. On subsequent test days, the tone-shock paired animals failed to exhibit fear CR to the CS. In contrast, animals that encountered a realistic agent of danger (a looming artificial owl) paired with a shock, simulating a plausible predatory strike, instantly fled to the nest when presented with a tone for the first time. These results highlight the possibility of a nonassociative, rather than standard associative, fear process providing survival function in life-threatening situations that animals are likely to encounter in nature.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Body temperature as a conditional response measure for pavlovian fear conditioning.Learn Mem. 2000 Sep-Oct;7(5):353-6. doi: 10.1101/lm.32800. Learn Mem. 2000. PMID: 11040267
-
Evidence of Pavlovian conditioned fear following electrical stimulation of the periaqueductal grey in the rat.Physiol Behav. 1987;40(1):55-63. doi: 10.1016/0031-9384(87)90185-5. Physiol Behav. 1987. PMID: 3615655
-
The amygdala is not necessary for unconditioned stimulus inflation after Pavlovian fear conditioning in rats.Learn Mem. 2009 Sep 30;16(10):645-54. doi: 10.1101/lm.1531309. Print 2009 Oct. Learn Mem. 2009. PMID: 19794190 Free PMC article.
-
The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats.J Neurosci. 2001 Mar 15;21(6):RC135. doi: 10.1523/JNEUROSCI.21-06-j0001.2001. J Neurosci. 2001. PMID: 11245704 Free PMC article.
-
Pavlovian or associative sensitization and its biological significance.Neurosci Biobehav Rev. 2024 Aug;163:105790. doi: 10.1016/j.neubiorev.2024.105790. Epub 2024 Jul 2. Neurosci Biobehav Rev. 2024. PMID: 38960076 Review.
Cited by
-
Fear conditioning in invertebrates.Front Behav Neurosci. 2022 Nov 10;16:1008818. doi: 10.3389/fnbeh.2022.1008818. eCollection 2022. Front Behav Neurosci. 2022. PMID: 36439964 Free PMC article. Review.
-
A top-down insular cortex circuit crucial for non-nociceptive fear learning.Sci Adv. 2025 May 9;11(19):eadt6996. doi: 10.1126/sciadv.adt6996. Epub 2025 May 9. Sci Adv. 2025. PMID: 40344067 Free PMC article.
-
How can ethology inform the neuroscience of fear, aggression and dominance?Nat Rev Neurosci. 2024 Dec;25(12):809-819. doi: 10.1038/s41583-024-00858-2. Epub 2024 Oct 14. Nat Rev Neurosci. 2024. PMID: 39402310 Review.
-
Investigating Neural Correlates of Behavior Through In Vivo Electrophysiology.Curr Protoc. 2023 May;3(5):e769. doi: 10.1002/cpz1.769. Curr Protoc. 2023. PMID: 37154436 Free PMC article.
-
Estimating foraging behavior in rodents using a modified paradigm measuring threat imminence dynamics.Neurobiol Stress. 2023 Nov 7;28:100585. doi: 10.1016/j.ynstr.2023.100585. eCollection 2024 Jan. Neurobiol Stress. 2023. PMID: 38024390 Free PMC article.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

