Buoyancy and hydrostatic balance in a West Indian Ocean coelacanth Latimeria chalumnae
- PMID: 35982432
- PMCID: PMC9389698
- DOI: 10.1186/s12915-022-01354-8
Buoyancy and hydrostatic balance in a West Indian Ocean coelacanth Latimeria chalumnae
Abstract
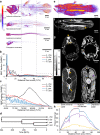
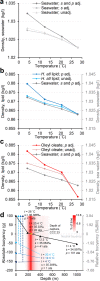
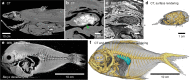
Background: Buoyancy and balance are important parameters for slow-moving, low-metabolic, aquatic organisms. The extant coelacanths have among the lowest metabolic rates of any living vertebrate and can afford little energy to keep station. Previous observations on living coelacanths support the hypothesis that the coelacanth is neutrally buoyant and in close-to-perfect hydrostatic balance. However, precise measurements of buoyancy and balance at different depths have never been made. RESULTS: Here we show, using non-invasive imaging, that buoyancy of the coelacanth closely matches its depth distribution. We found that the lipid-filled fatty organ is well suited to support neutral buoyancy, and due to a close-to-perfect hydrostatic balance, simple maneuvers of fins can cause a considerable shift in torque around the pitch axis allowing the coelacanth to assume different body orientations with little physical effort.
Conclusions: Our results demonstrate a close match between tissue composition, depth range and behavior, and our collection-based approach could be used to predict depth range of less well-studied coelacanth life stages as well as of deep sea fishes in general.
Keywords: Bone mineral density; Computed tomography; Depth regulation; Ecophysiology; Fatty organ; Headstand; Lipid accumulation; Magnetic resonance imaging; Magnetic resonance spectroscopy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Erdmann MV, Caldwell RL, Kasim Moosa M. Indonesian ‘king of the sea’ discovered. Nature. 1998;395:335. doi: 10.1038/26376. - DOI
-
- Forey PL. History of the Coelacanth Fishes. London, United Kingdom: Chapman and Hall; 1998.
-
- Hissmann K, Fricke H, Schauer J. Patterns of time and space utilisation in coelacanths (Latimeria chalumnae), determined by ultrasonic telemetry. Mar Biol. 2000;136:943–952. doi: 10.1007/s002270000294. - DOI
-
- Hissmann K, Fricke H, Schauer J, Ribbink AJ, Roberts M, Sink K, Heemstra PC. The South African coelacanths – an account of what is known after three submersible expeditions. S Afr J Sci. 2006;102:491–500.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

