This is a preprint.
Viral and Symptom Rebound in Untreated COVID-19 Infection
- PMID: 35982660
- PMCID: PMC9387151
- DOI: 10.1101/2022.08.01.22278278
Viral and Symptom Rebound in Untreated COVID-19 Infection
Abstract
Background: There are reports of viral RNA and symptom rebound in people with COVID-19 treated with nirmatrelvir/ritonavir. Since the natural course of viral and symptom trajectories of COVID-19 has not been well described, we evaluated the incidence of viral and symptom rebound in untreated outpatients with mild-moderate COVID-19.
Methods: The study population included 568 participants enrolled in the ACTIV-2/A5401 platform trial who received placebo. Anterior nasal swabs were collected for SARS-CoV-2 RNA testing on days 0-14, 21 and 28. Participants recorded the severity of 13 targeted symptoms daily from day 0 to 28. Viral rebound was defined as ≥0.5 log10 viral RNA copies/mL increase and symptom rebound was defined as a 4-point total symptom score increase from baseline. Baseline was defined as study day 4 (primary analysis) or 8 days from symptom onset (secondary analysis).
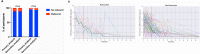
Findings: In both the primary and secondary analyses, 12% of participants had viral rebound. Viral rebounders were older than non-rebounders (median 54 vs 47 years, P=0.04). Symptom rebound occurred in 27% of participants after initial symptom improvement and in 10% of participants after initial symptom resolution. The combination of high-level viral rebound to ≥5.0 log10 RNA copies/mL and symptom rebound after initial improvement was observed in 1-2% of participants.
Interpretation: Viral RNA rebound or symptom relapse in the absence of antiviral treatment is common, but the combination of high-level viral and symptom rebound is rare.
Keywords: COVID-19; Paxlovid; symptom rebound; viral rebound.
Conflict of interest statement
Declaration of interests KWC has received research funding to the institution from Merck Sharp & Dohme and is a consultant for Pardes Bioscences. ESD has consulted for Gilead, Merck and ViiV and received research support from Gilead and ViiV. JZL has consulted for Abbvie and received research funding from Merck. JSC has consulted for Merck & Company. ALG reports contract testing from Abbott, Cepheid, Novavax, Pfizer, Janssen and Hologic and research support from Gilead and Merck, outside of the described work. JJE has consulted for GSK, and Merck. DAW has consulted for Gilead and ViiV and received research support from Gilead, ViiV, and Lilly.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
