ALKBH5/MAP3K8 axis regulates PD-L1+ macrophage infiltration and promotes hepatocellular carcinoma progression
- PMID: 35982895
- PMCID: PMC9379398
- DOI: 10.7150/ijbs.70149
ALKBH5/MAP3K8 axis regulates PD-L1+ macrophage infiltration and promotes hepatocellular carcinoma progression
Abstract
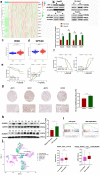
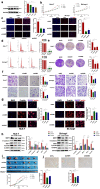
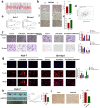
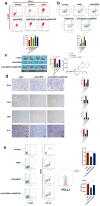
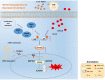
Hepatocellular carcinoma is one of the most common malignant tumors.M6A is a novel epigenetic modification that have been emerged as vital regulators for the progression of HCC. However, the regulatory role, clinical significance and the details of the modification, such as the impact on the local tumor environment, remain largely unclear. Our study showed that ALKBH5 was highly expressed in HCC and high ALKBH5 expression predicted a worse prognosis of HCC patients. Prediction of ALKBH5 function by tissue samples and single cell sequencing Gene Set Variation Analysis. Primary CD3 + T lymphocytes and bone marrow-derived macrophages were used to evaluate the effect of ALKBH5 on immune microenvironment. The results indicated that ALKBH5 promote HCC cell proliferation, metastasis and PD-L1+macrophage recruitment. Mechanistically the results showed that ALKBH5 regulates MAP3K8 expression in a m6A dependent manner which mediates the proliferation and metastasis of HCC cells. ALKBH5 also promotes the activation of JNK and ERK pathways through upregulating MAP3K8, thus regulating the expression of IL-8 and promoting macrophage recruitment. Taken together, these data show that ALKBH5 promotes HCC growth, metastasis and macrophage recruitment through ALKBH5/MAP3K8 axis and it may serve as a potential diagnostic marker and target for treatment of HCC patients.
Keywords: Hypoxia; Immune microenvironment; M6A; PD-L1; Tumor-associated macrophages.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Bray F, Ferlay J, Soerjomataram I. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

