Survival and adverse events of elderly patients treated with sorafenib for hepatocellular carcinoma
- PMID: 35982971
- PMCID: PMC9380437
- DOI: 10.3389/fonc.2022.829483
Survival and adverse events of elderly patients treated with sorafenib for hepatocellular carcinoma
Abstract
Introduction: The first-line treatment for advanced hepatocellular carcinoma (HCC) is atezolizumab plus bevacizumab, but its availability is not universal and elderly patients are underrepresented in clinical trials. There is little evidence of efficacy and tolerability in elderly patients under systemic treatment. The aims of this study were to characterize the profile of elderly patients treated with sorafenib, assess their survival and safety profile in order to extrapolate their eligibility for systemic treatment.
Methods: Retrospective multicentre study of HCC patients aged ≥75 years old treated with sorafenib from January 2008 to December 2019. Demographic data, baseline characteristics, and variables related to HCC and sorafenib were recorded. Overall survival (OS) and safety were analyzed.
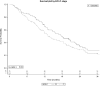
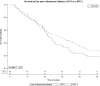
Results: The study included 206 patients from 11 hospitals, median age 77.9 years; 71.4% men and 62.6% stage Barcelona Clinic Liver Cancer- C (BCLC-C). The main causes of cirrhosis were hepatitis C (60.7%) and alcohol (14.7%). Most patients (84.5%) started with sorafenib 800mg and 15.5% at lower dosage. Arterial hypertension (AHT) (74.2 vs 62.2%; standardized mean differences (STD): 26) and baseline ECOG-PS>0 (45.3 vs 34.7%; STD: 38.2) differed significantly between patients receiving low and full doses. Median OS was 15.4 months (18.2 in BCLC-B vs 13.6 in BCLC-C). OS was not modified by comorbidities, age or period with more expertise.
Conclusions: Sorafenib appears to be safe in elderly patients with HCC. This is the first study to characterize the profile of elderly patients to be considered for systemic treatment. These findings could be used as the reference profile for elderly candidates for atezolizumab-bevacizumab.
Keywords: elderly patients; hepatocellular carcinoma; outcome; overall survival; safety; sorafenib.
Copyright © 2022 Soria, Calvo, Casas, Vidales, Muñoz-Martínez, Sapena, Puigvehi, Canillas, Guardeño, Gallego, Mínguez, Horta, Clos, Montoliu, Roget, Reig and Vergara.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. . Efficacy and safety of sorafenib in patients in the Asia-pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol (2009) 10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials