Long-term follow-up of early stage HER2-positive breast cancer patients treated with trastuzumab: A population-based real world multicenter cohort study
- PMID: 35982977
- PMCID: PMC9379250
- DOI: 10.3389/fonc.2022.861324
Long-term follow-up of early stage HER2-positive breast cancer patients treated with trastuzumab: A population-based real world multicenter cohort study
Abstract
Introduction: Since its introduction in standard of care, trastuzumab has revolutionized the treatment of patients with early and late stages of HER2-positive breast cancer. While the initial clinical trials were convincing and lead to major changes in practice, more knowledge on the long-term outcome and tolerability is needed. The present study was designed to assess the survival, prognostic factors and relapse patterns after the implementation of trastuzumab in a real-world cohort.
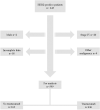
Methods: All cases of HER2-positive breast cancer diagnosed between 2006 and 2014 in the Southeast Healthcare Region of Sweden were retrospectively identified. Medical records were thoroughly reviewed with regard to clinicopathological parameters, treatments, relapse pattern and adverse events.
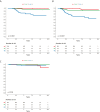
Results: 643 patients were identified and 599 were eligible for analysis. Breast cancer specific survival, distant recurrence free survival and local recurrence free survival were 93.4%, 89.7% and 98.0% for trastuzumab treated patients and 87.4%, 81.6% and 87.4% in patients not treated with trastuzumab, respectively. ER status, nodal status and trastuzumab treatment were all independent prognostic factors in multivariable analysis. No new safety concerns were discovered.
Conclusion: The real-world outcome of trastuzumab-treated patients with early HER2-positive breast cancer is similar to what has been previously reported in long-term follow up of prospective clinical trials. ER status, nodal status and trastuzumab treatment are independent prognostic factors for breast cancer specific mortality rate, distant recurrence rate and locoregional recurrence rate in HER2-positive patients in the trastuzumab era.
Keywords: HER2; adjuvant; breast cancer; prognostic factors; real-world evidence; trastuzumab (Herceptin).
Copyright © 2022 Ellegård, Engvall, Asowed, Hallbeck, Elander and Stål.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Breast cancer . Available at: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (Accessed May 27, 2021).
-
- Rydén L, Haglund M, Bendahl P-O, Hatschek T, Kolaric A, Kovács A, et al. . Reproducibility of human epidermal growth factor receptor 2 analysis in primary breast cancer: a national survey performed at pathology departments in Sweden. Acta Oncol (2009) 48:860–6. doi: 10.1080/02841860902862511 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

