PD-L1 expression on circulating tumor cells can be a predictive biomarker to PD-1 inhibitors combined with radiotherapy and antiangiogenic therapy in advanced hepatocellular carcinoma
- PMID: 35982979
- PMCID: PMC9379259
- DOI: 10.3389/fonc.2022.873830
PD-L1 expression on circulating tumor cells can be a predictive biomarker to PD-1 inhibitors combined with radiotherapy and antiangiogenic therapy in advanced hepatocellular carcinoma
Abstract
Aim: A programmed death 1 (PD-1) inhibitor coupled with radiotherapy and antiangiogenic therapy is a potential therapeutic strategy for advanced hepatocellular carcinoma (HCC). We aimed to determine if circulating tumor cells (CTCs) positive for programmed death-ligand 1 (PD-L1) could be employed as a predictive biomarker in HCC patients receiving triple therapy.
Methods: In this study, HCC patients received a PD-1 inhibitor in combination with intensity-modulated radiotherapy (IMRT) and antiangiogenic therapy. Following IMRT, the PD-1 inhibitor was administrated once every 3 weeks, while the antiangiogenic drug was given once a day. Treatment was continued until the disease progressed. Two mL of peripheral blood was collected at baseline, 1 month, and 3 months after treatment for CTC enrichment using the CytoSorter® system with a CytoSorter™ CTC PD-L1 Kit (Watson Biotech., China).
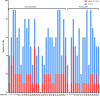
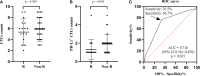
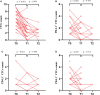
Result: A total of 47 HCC patients receiving the triple therapy were enrolled in this study. Patients with < 2 PD-L1+ CTCs at baseline had a higher objective response rate (ORR) and longer overall survival (OS) than those with ≥ 2 PD-L1+ CTCs (56.5% vs. 16.7%, p = 0.007; not reach vs. 10.8 months, p = 0.001, respectively). The count of PD-L1+ CTCs was found to be an independent predictive biomarker of OS. Furthermore, the objective response was more likely to be achieved in patients with a dynamic decrease in PD-L1+ CTC counts at 1 month after treatment.
Conclusions: Our study demonstrated that PD-L1+ CTCs could be a predictive biomarker for HCC patients receiving PD-1 inhibitors in combination with IMRT and antiangiogenic therapy.
Keywords: antiangiogenic therapy; circulating tumor cells; hepatocellular carcinoma; programmed death 1 inhibitor; programmed death-ligand 1; radiotherapy.
Copyright © 2022 Su, Guo, He, Rao, Zhang, Yang, Huang, Gu, Xu, Liu, Wang, Chen, Wu, Hu, Zeng, Li, Tong, Li, Yang, Liu, Xu, Tan, Tang, Feng, Chen, Yang, Jin, Zhu, Li and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Medical
Research Materials

