Substituted Cyclopentannulated Tetraazapentacenes
- PMID: 35983676
- PMCID: PMC9826220
- DOI: 10.1002/chem.202201842
Substituted Cyclopentannulated Tetraazapentacenes
Abstract
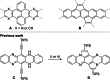
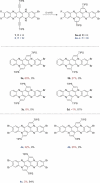
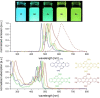
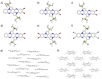
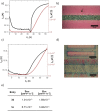
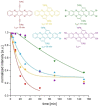
Brominated pentannulated dihydrotetraazapentacenes were prepared by gold- or palladium-catalyzed 5-endo-dig cyclization of TIPS-ethynylated dihydrotetraazaacenes (TIPS = triisopropylsilyl). Post-functionalization was demonstrated by Sonogashira alkynylation and Rosenmund-von Braun cyanation. Calculations predict these species to act as n-type semiconductors, which was verified for two derivates through characterization in organic field-effect transistors.
Keywords: cyclization; dihydroazaacenes; postfunctionalization; semiconductors; solid-state packing.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

