Pas de Deux of an NO Couple: Synchronous Photoswitching from a Double-Linear to a Double-Bent Ru(NO)2 Core under Nitrosyl Charge Conservation
- PMID: 35983847
- PMCID: PMC9826364
- DOI: 10.1002/anie.202210671
Pas de Deux of an NO Couple: Synchronous Photoswitching from a Double-Linear to a Double-Bent Ru(NO)2 Core under Nitrosyl Charge Conservation
Abstract
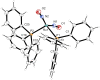
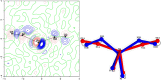
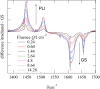
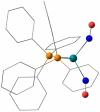
The {Ru(NO)2 }10 dinitrosylruthenium complex [Ru(NO)2 (PPh3 )2 ] (1) shows photo-induced linkage isomerism (PLI) of a special kind: the two NO ligands switch, on photo-excitation, synchronously from the ground state (GS) with two almost linear RuNO functions to a metastable state (MS) which persists up to 230 K and can be populated to ≈50 %. The MS was experimentally characterised by photo-crystallography, IR spectroscopy and DS-calorimetry as a double-bent variant of the double-linear GS. The experimental results are confirmed by computation which unravels the GS/MS transition as a disrotatory synchronous 50° turn of the two nitrosyl ligands. Although 1 shows the usual redshift of the N-O stretch on bending the MNO unit, there is no increased charge transfer from Ru to NO along the GS-to-MS path. In terms of the effective-oxidation-state (EOS) method, both isomers of 1 and the transition state are Ru-II (NO+ )2 species.
Keywords: Coordination Modes; Nitrosyl Ligands; Photo-Induced Linkage Isomerism; Photophysics; Ruthenium.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- None
-
- Feringa B. L., Angew. Chem. Int. Ed. 2017, 56, 11060–11078; - PubMed
- Angew. Chem. 2017, 129, 11206–11226;
-
- Bléger D., Hecht S., Angew. Chem. Int. Ed. 2015, 54, 11338–11349; - PubMed
- Angew. Chem. 2015, 127, 11494–11506;
-
- McConnell A. J., Wood C. S., Neelakandan P. P., Nitschke J. R., Chem. Rev. 2015, 115, 7729–7793. - PubMed
-
- None
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

