Availability of Results of Trials Studying Pancreatic Adenocarcinoma over the Past 10 Years
- PMID: 35983949
- PMCID: PMC9632316
- DOI: 10.1093/oncolo/oyac156
Availability of Results of Trials Studying Pancreatic Adenocarcinoma over the Past 10 Years
Abstract
Background: Pancreatic adenocarcinoma (PDAC) is a lethal cancer with few therapeutic options. Availability of results is a crucial step in interventional research. Our aim was to evaluate results availability for trials in patients with PDAC and explore associated factors.
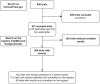
Materials and methods: We performed a retrospective cohort study and searched the ClinicalTrials.gov registry for trials evaluating PDAC management with a primary completion date between 1 January 2010 and 1 June 2020. Then, we searched for results submitted on ClinicalTrials.gov and/or published. Our primary outcome was the proportion of PDAC trials with available results: submitted on ClinicalTrials.gov (either publicly available or undergoing quality control check) and/or published in a full-text article. The association of predefined trial characteristics with results availability was assessed.
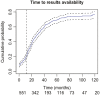
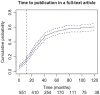
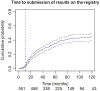
Results: We identified 551 trials of which 386 (70%) had available results. The cumulative percentage of trials with available results was 21% (95% CI, 18-25%) at 12 months after the primary completion date, 44% (95% CI, 30-48%) at 24 months and 57% (95% CI, 53-61%) at 36 months. Applicable clinical trials, required to comply with the 2007 Food and Drug Administration Amendments Act 801 and its final rule on reporting of results on ClinicalTrials.gov, were more likely to have available results over time (HR 2.1 [95% CI 1.72-2.63], P < .001). Industry-funded, small sample size, and terminated trials were less likely to have available results. Other trial characteristics showed no association with results availability.
Conclusion: Our results highlight a waste in interventional research studying PDAC.
Keywords: ClinicalTrials.gov; FDAAA 801; interventional research; pancreatic adenocarcinoma; results availability.
© The Author(s) 2022. Published by Oxford University Press.
Figures
Similar articles
-
Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study.Lancet. 2020 Feb 1;395(10221):361-369. doi: 10.1016/S0140-6736(19)33220-9. Epub 2020 Jan 17. Lancet. 2020. PMID: 31958402
-
Availability of results of interventional studies assessing colorectal cancer from 2013 to 2020.PLoS One. 2022 Apr 11;17(4):e0266496. doi: 10.1371/journal.pone.0266496. eCollection 2022. PLoS One. 2022. PMID: 35404939 Free PMC article.
-
Compliance with results reporting at ClinicalTrials.gov.N Engl J Med. 2015 Mar 12;372(11):1031-9. doi: 10.1056/NEJMsa1409364. N Engl J Med. 2015. PMID: 25760355 Free PMC article.
-
Multicenter randomized controlled trial and registry study to assess the safety and efficacy of the NanoKnife® system for the ablation of stage 3 pancreatic adenocarcinoma: overview of study protocols.BMC Cancer. 2021 Jul 7;21(1):785. doi: 10.1186/s12885-021-08474-4. BMC Cancer. 2021. PMID: 34233640 Free PMC article. Review.
-
Association of the FDA Amendment Act with trial registration, publication, and outcome reporting.Trials. 2017 Jul 18;18(1):333. doi: 10.1186/s13063-017-2068-3. Trials. 2017. PMID: 28720112 Free PMC article. Review.
Cited by
-
Cellular collusion: cracking the code of immunosuppression and chemo resistance in PDAC.Front Immunol. 2024 May 16;15:1341079. doi: 10.3389/fimmu.2024.1341079. eCollection 2024. Front Immunol. 2024. PMID: 38817612 Free PMC article. Review.
-
CONSORT 2025 explanation and elaboration: updated guideline for reporting randomised trials.BMJ. 2025 Apr 14;389:e081124. doi: 10.1136/bmj-2024-081124. BMJ. 2025. PMID: 40228832 Free PMC article.
-
Early phase trials: the perils of nonpublication.Oncologist. 2025 Apr 4;30(4):oyaf041. doi: 10.1093/oncolo/oyaf041. Oncologist. 2025. PMID: 40271638 Free PMC article. No abstract available.
-
Therapeutic developments in pancreatic cancer.Nat Rev Gastroenterol Hepatol. 2024 Jan;21(1):7-24. doi: 10.1038/s41575-023-00840-w. Epub 2023 Oct 5. Nat Rev Gastroenterol Hepatol. 2024. PMID: 37798442 Review.
-
Time to publication for results of clinical trials.Cochrane Database Syst Rev. 2024 Nov 27;11(11):MR000011. doi: 10.1002/14651858.MR000011.pub3. Cochrane Database Syst Rev. 2024. PMID: 39601300
References
-
- Bray F, Ferlay J, Soerjomataram I, et al. . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

