Availability of Results of Trials Studying Pancreatic Adenocarcinoma over the Past 10 Years
- PMID: 35983949
- PMCID: PMC9632316
- DOI: 10.1093/oncolo/oyac156
Availability of Results of Trials Studying Pancreatic Adenocarcinoma over the Past 10 Years
Abstract
Background: Pancreatic adenocarcinoma (PDAC) is a lethal cancer with few therapeutic options. Availability of results is a crucial step in interventional research. Our aim was to evaluate results availability for trials in patients with PDAC and explore associated factors.
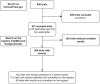
Materials and methods: We performed a retrospective cohort study and searched the ClinicalTrials.gov registry for trials evaluating PDAC management with a primary completion date between 1 January 2010 and 1 June 2020. Then, we searched for results submitted on ClinicalTrials.gov and/or published. Our primary outcome was the proportion of PDAC trials with available results: submitted on ClinicalTrials.gov (either publicly available or undergoing quality control check) and/or published in a full-text article. The association of predefined trial characteristics with results availability was assessed.
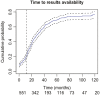
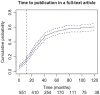
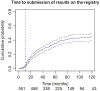
Results: We identified 551 trials of which 386 (70%) had available results. The cumulative percentage of trials with available results was 21% (95% CI, 18-25%) at 12 months after the primary completion date, 44% (95% CI, 30-48%) at 24 months and 57% (95% CI, 53-61%) at 36 months. Applicable clinical trials, required to comply with the 2007 Food and Drug Administration Amendments Act 801 and its final rule on reporting of results on ClinicalTrials.gov, were more likely to have available results over time (HR 2.1 [95% CI 1.72-2.63], P < .001). Industry-funded, small sample size, and terminated trials were less likely to have available results. Other trial characteristics showed no association with results availability.
Conclusion: Our results highlight a waste in interventional research studying PDAC.
Keywords: ClinicalTrials.gov; FDAAA 801; interventional research; pancreatic adenocarcinoma; results availability.
© The Author(s) 2022. Published by Oxford University Press.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, et al. . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

