Visualizations throughout pharmacoepidemiology study planning, implementation, and reporting
- PMID: 35984046
- PMCID: PMC9826437
- DOI: 10.1002/pds.5529
Visualizations throughout pharmacoepidemiology study planning, implementation, and reporting
Abstract
Transparency is increasingly promoted to instill trust in nonrandomized studies using real-world data. Graphics and data visualizations support transparency by aiding communication and understanding, and can inform study design and analysis decisions. However, other than graphical representation of a study design and flow diagrams (e.g., a Consolidated Standards of Reporting Trials [CONSORT] like diagram), specific standards on how to maximize validity and transparency with visualization are needed. This paper provides guidance on how to use visualizations throughout the life cycle of a pharmacoepidemiology study-from initial study design to final report-to facilitate rationalized and transparent decision-making about study design and implementation, and clear communication of study findings. Our intent is to help researchers align their practices with current consensus statements on transparency.
Keywords: data visualizations; graphs; pharmacoepidemiology.
© 2022 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Gatto, Ms. Mattox and Dr. Rassen are employees of Aetion, Inc., with stock options or existing equity. Dr. Gatto also owns stock in Pfizer Inc. Dr. Wang receives salary support as PI for investigator‐initiated grants to Brigham and Women's Hospital from Novartis, Johnson & Johnson, and Boehringer Ingelheim and is a consultant to Aetion for unrelated work. She is also PI on grants from the National Institute of Aging, Laura and John Arnold Foundation and the FDA Sentinel Initiative. She co‐directs the REPEAT Initiative, a non‐profit program with projects focused on increasing transparency and reproducibility of database studies. Dr. Murk is a contractor of and owns shares in Aetion, Inc., a technology company that provides analytic software and services to the healthcare industry. Dr. Brookhart serves on scientific advisory committees for Amgen, Astellas/Seagen, Atara Biotherapeutics, Brigham and Women's Hospital, Gilead, Kite, NIDDK, and Vertex; he receives consulting fees and owns equity in Target RWE. Dr. Bate is a full‐time employee of GSK and holds stock and stock options at Pfizer and GSK. Dr. Schneeweiss is participating in investigator‐initiated grants to the Brigham and Women's Hospital from Boehringer Ingelheim and UCB unrelated to the topic of this study. He is a consultant to Aetion Inc., a software manufacturer of which he owns equity. He is an advisor to Temedica GmbH, a patient‐oriented data generation company. His interests were declared, reviewed, and approved by the Brigham and Women's Hospital in accordance with their institutional compliance policies.
Figures



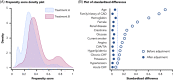
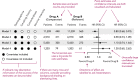

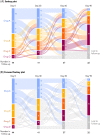
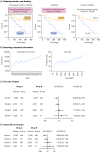
References
-
- Orsini LS, Berger M, Crown W, et al. Improving transparency to build trust in real‐world secondary data studies for hypothesis testing—why, what, and how: recommendations and a road map from the real‐world evidence transparency initiative. Value Health. 2020;23(9):1128‐1136. doi:10.1016/j.jval.2020.04.002 - DOI - PubMed
-
- Wainer H. How to display data badly. Am Stat. 1984;38(2):137‐147. doi:10.1080/00031305.1984.10483186 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

