Tissue attenuation imaging and tissue scatter imaging for quantitative ultrasound evaluation of hepatic steatosis
- PMID: 35984128
- PMCID: PMC9387959
- DOI: 10.1097/MD.0000000000029708
Tissue attenuation imaging and tissue scatter imaging for quantitative ultrasound evaluation of hepatic steatosis
Abstract
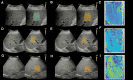
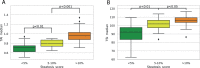
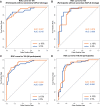
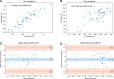
We aimed to assess the feasibility of ultrasound-based tissue attenuation imaging (TAI) and tissue scatter distribution imaging (TSI) for quantification of liver steatosis in patients with nonalcoholic fatty liver disease (NAFLD). We prospectively enrolled 101 participants with suspected NAFLD. The TAI and TSI measurements of the liver were performed with a Samsung RS85 Prestige ultrasound system. Based on the magnetic resonance imaging proton density fat fraction (MRI-PDFF), patients were divided into ≤5%, 5-10%, and ≥10% of MRI-PDFF groups. We determined the correlation between TAI, TSI, and MRI-PDFF and used multiple linear regression analysis to identify any association with clinical variables. The diagnostic performance of TAI, TSI was determined based on the area under the receiver operating characteristic curve (AUC). The intraclass correlation coefficient (ICC) was calculated to assess interobserver reliability. Both TAI (rs = 0.78, P < .001) and TSI (rs = 0.68, P < .001) showed significant correlation with MRI-PDFF. TAI overperformed TSI in the detection of both ≥5% MRI-PDFF (AUC = 0.89 vs 0.87) and ≥10% (AUC = 0.93 vs 0.86). MRI-PDFF proved to be an independent predictor of TAI (β = 1.03; P < .001), while both MRI-PDFF (β = 50.9; P < .001) and liver stiffness (β = -0.86; P < .001) were independent predictors of TSI. Interobserver analysis showed excellent reproducibility of TAI (ICC = 0.95) and moderate reproducibility of TSI (ICC = 0.73). TAI and TSI could be used successfully to diagnose and estimate the severity of hepatic steatosis in routine clinical practice.
Copyright © 2022 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
P.N.K. has served as a speaker for Samsung Medison Ltd. All other authors declare no conflict of interest.
Figures

References
-
- Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol. 2019;70:151–71. - PubMed
-
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
-
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

