Modeling the physiological roles of the heart and kidney in heart failure with preserved ejection fraction during baroreflex activation therapy
- PMID: 35984764
- PMCID: PMC9467477
- DOI: 10.1152/ajpheart.00329.2022
Modeling the physiological roles of the heart and kidney in heart failure with preserved ejection fraction during baroreflex activation therapy
Abstract
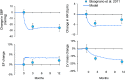
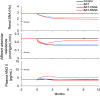
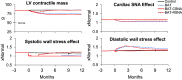
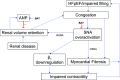
Heart failure (HF) is a leading cause of death and is increasing in prevalence. Unfortunately, therapies that have been efficacious in patients with HF with reduced ejection fraction (HFrEF) have not convincingly shown a reduction in cardiovascular mortality in patients with HF with preserved ejection fraction (HFpEF). It is thought that high sympathetic nerve activity (SNA) in the heart plays a role in HF progression. Clinical trials demonstrate that baroreflex activation therapy reduces left ventricular (LV) mass and blood pressure (BP) in patients with HFpEF and hypertension; however, the mechanisms are unclear. In the present study, we used HumMod, a large physiology model to simulate HFpEF and predict the time-dependent changes in systemic and cardiac hemodynamics, SNA, and cardiac stresses during baroreflex activation. The baseline HFpEF model was associated with elevations in systolic BP, diastolic dysfunction, and LV hypertrophy and stiffness similar to clinical HFpEF. Simulating 12 mo of baroreflex activation resulted in reduced systolic BP (-25 mmHg) and LV mass (-15%) similar to clinical evidence. Baroreflex activation also resulted in sustained decreases in cardiac and renal SNA (-22%) and improvement in LV β1-adrenergic function. However, the baroreflex-induced reductions in BP and improvements in cardiac stresses, mass, and function were mostly attenuated when renal SNA was clamped at baseline levels. These simulations suggest that the suppression of renal SNA could be a primary determinant of the cardioprotective effects from baroreflex activation in HFpEF.NEW & NOTEWORTHY Treatments that are efficacious in patients with HFrEF have not shown a significant impact on cardiovascular mortality in patients with HFpEF. We believe these simulations offer novel insight into the important roles of the cardiac and renal nerves in HFpEF and the potential mechanisms of how baroreflex activation alleviates HFpEF disease progression.
Keywords: HFpEF; baroreflex activation; heart failure; physiological model.
Conflict of interest statement
W.A.P. is the CSO for HC Simulation, LLC, which does not alter the authors’ adherence to all the American Physiological Society’s policies on sharing data and materials.
Figures






References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

